Air - composition and properties pt 2
how to present evidence for the existence of air;
how to plan and carry out experiments confirming that the air is a mixture;
how to describe the air composition.
to present a short history of discoveries related to the air;
how to describe air properties;
to list examples of the use of gases forming air in everyday life;
to plan and conduct experiments to examine the basic properties of air;
to safely use laboratory equipment and chemical reagents.
Properties and applications of air
Find information on what the research was done to discover air on the Internet, a textbook and e‑textbook. Look at the timeline where the greatest discoveries associated with this gas mixture were marked.
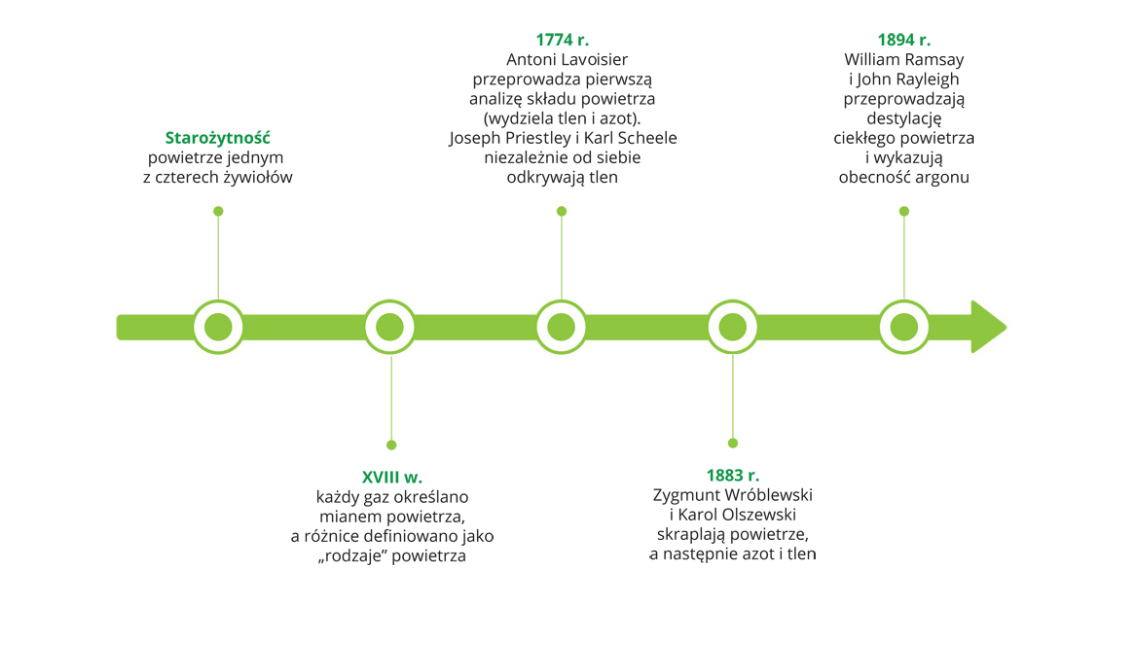
1. Ancient times wind as one of four elements
2. 8th century each gas was called air and the differences were defined as "types" of air
3. 1774 r. Antoine Lavoisier carries out the first analysis of the composition of the air (separated oxygen and nitrogen). Joseph Priestley and Karl Scheele independently discover oxygen.
4. 1883 r. Zygmunt Wróblewski and Karol Olszewski condense the air, and then nitrogen and oxygen
5. 1894 r. William Ramsay and John Rayleigh perform the distillation of liquid air and show the presence of argon.
Expand the following points and read the information about the air.
Physical properties of the mixture
The air is a homogeneous mixture of gases. Colourless, odourless, tasteless, slightly soluble in water.
Liquefaction
Under conditions of low temperatures and significantly increased pressure, the gases from the air can be condensed. The components of the liquid air have different boiling points, so that these can be separated by distillation. For the first time, nitrogen and oxygen were condensed in 1883 by Polish scientists: chemist Karol Olszewski and physicist Zygmunt Wróblewski. The condensed air takes on a pale blue colour, and its density is lower than the density of water.
Density
The air density depends on the pressure, temperature and composition. At atmospheric pressure, at sea level, at 0°C, the air density is 1.3 kg/m3.
Application
The air is necessary for the life of man and other organisms on Earth. In the industry, these are used in combustion processes and as a raw material for the production of oxygen, nitrogen, argon and noble gases.
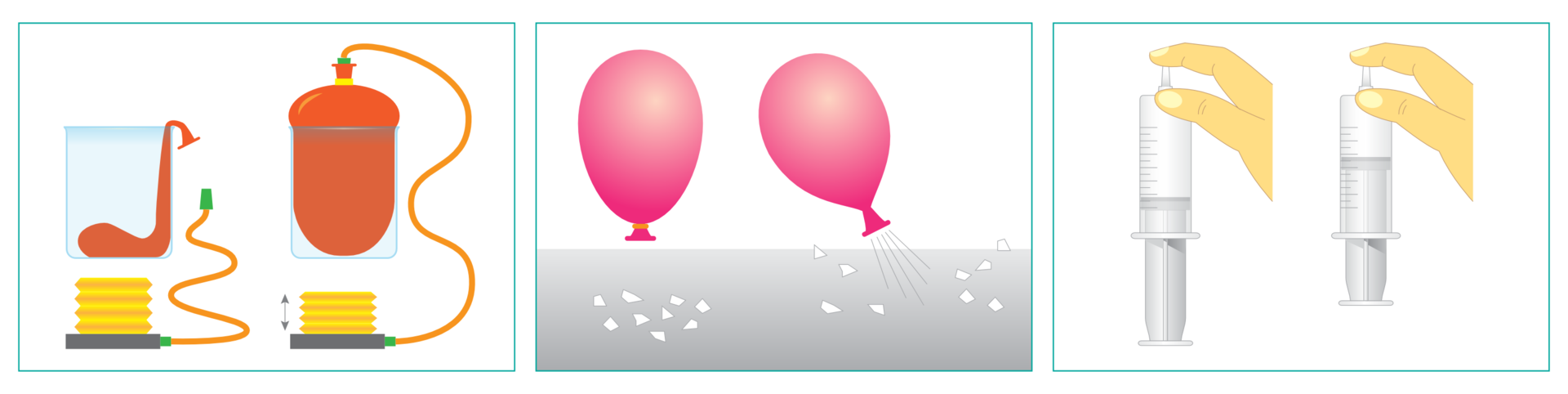
Before you perform the experiment „Testing properties of the air” write down the research question and select the hypothesis. Note down observations and conclusions as well.
Select one of the presented hypotheses, and then verify it.
The air takes on the shape of the vessel in which it is located.
The air cannot be compressed.
a glass,
balloon,
pump,
scraps of paper,
syringe.
I.
Put an empty balloon on the bottom of the glass and start pumping it with air.
Observe the changes.
II.
Fill in the balloon with air.
Place scraps of paper on the table.
Let the air out of the balloon.
Observe the changes taking place.
III.
Pull out the syringe plunger.
Plug the hole of the syringe with your finger and press on the plunger.
Pull the piston up.
Observe the changes taking place.
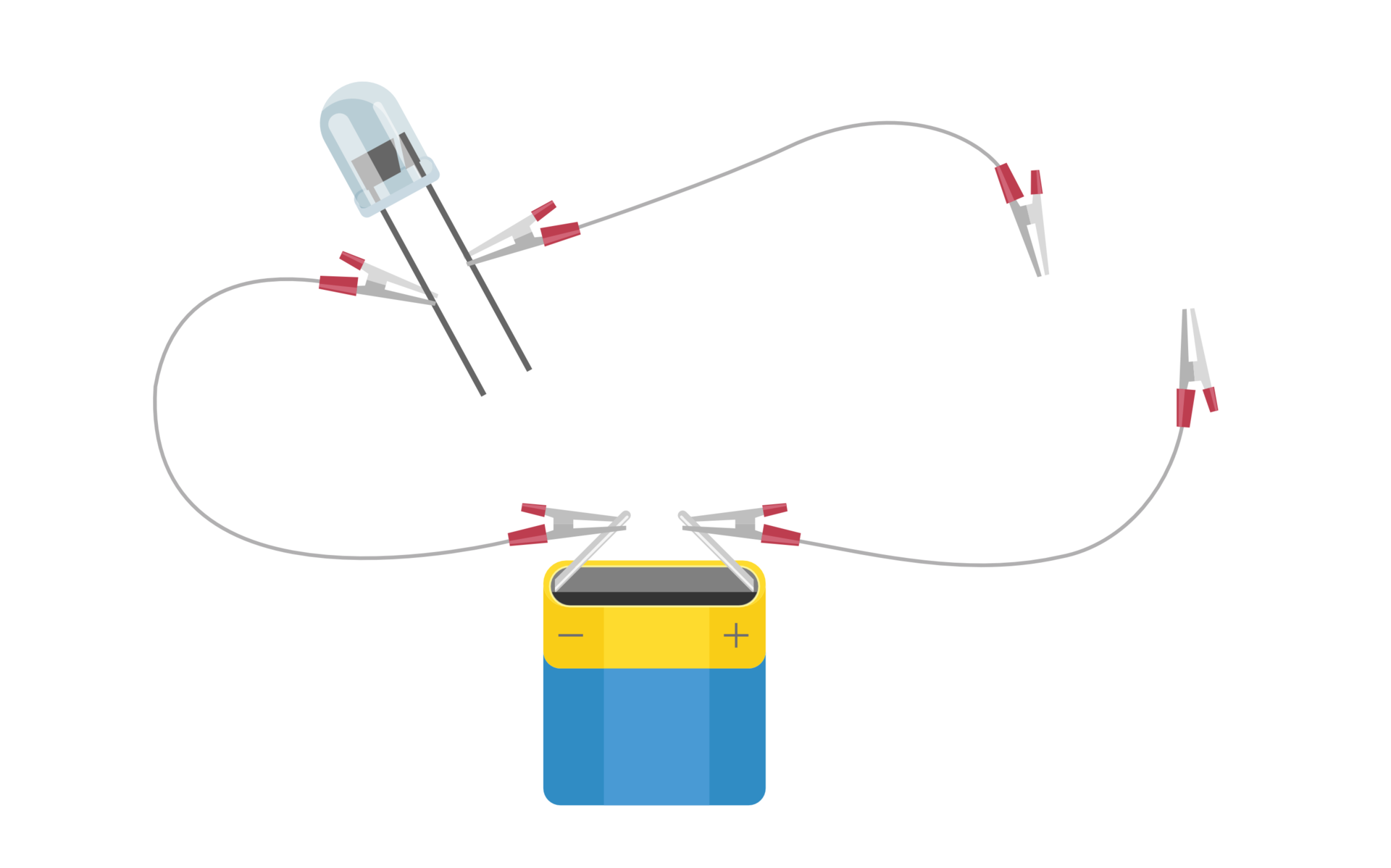
Before you perform the experiment “Testing electrical conductivity of air” write down the research question and select the hypothesis. Note down observations and conclusions as well.
Select one hypothesis and then verify it.
The air conducts electricity.
The air does not conduct electricity.
battery,
light bulb,
electric wires.
Leave the ends of the set with the battery and the bulb unplugged.
Observe what is happening with the bulb.
Read the descriptions and confirm or deny them. Flip the card and see if your answer is correct.
| The air is blue. | No - it is colourless |
| Nitrogen accounts for half of the air composition. | No - over 70% |
| The air is a mixture of colourless and odourless gases. | Yes - nitrogen, oxygen, argon and other noble gases |
| There is water in the air. | Yes - in the form of water vapour |
| The air is conducts electricity | No. |
| The air is not compressible. | It is - you can easily reduce or increase its volume |
| The air can be easily dissolved in water | No - is slightly soluble in water |
Check the answer if all the drawn elements will describe the proper use of gases.
|
argon
|
breathing mixes in air tanks
|

|
|
oxygen
|
foam and powder extinguishers
|

|
|
carbon dioxide
|
is used in metallurgy, including for steel production
|

|
|
nitrogen
|
filling in computer hard drives
|

|
Homogeneous mixture of various substances, mainly gases, without color, taste and smell, constituting the Earth's atmosphere is called:
- air
- neon
- nitrogen
Summary
The air is a homogeneous mixture of colourless and odourless gases.
The main components of the air are: nitrogen (78%), oxygen (21%), argon and other noble gases (0.94%), carbon dioxide and water in the form of steam.
For the first time, the air was liquefied by Polish chemists: Karol Olszewski and Zygmunt Wróblewski.
The air is compressible – you can easily reduce its volume. You can also expand them, or increase its volume.
The air does not conduct electricity.
Keywords
air, oxygen, nitrogen, noble gases, atmosphere, homogeneous mixture
Glossary
powietrze – jednorodna mieszanina różnych substancji, głównie gazów, bez barwy, smaku i zapachu, stanowiąca atmosferę ziemską