Alcohols - properties
how monohydric alcohols are built;
how to draw a structural formula of methanol and ethanol;
how to create alcohol names;
what is the valence of carbon in organic compounds.
distinguish methanol from ethanol based on physical properties;
write the equation for the combustion reaction of alcohols.
Physical properties of methanol and ethanol
Methanol and ethanol are colourless liquids that dissolve well in water. Therefore, these cannot be distinguished without laboratory tests, e.g. determination of boiling point or density. In trade, concentrated ethyl alcohol is called grain alcohol.

1. rectified spirit
2. ethyl alcohol completely contaminated
3. methanol
As we know, the process of alcohol fermentation occurs under the influence of a special species of yeast found in the peels of ripe grapes (or other fruits). Glucose – sugar contained in fruit juice – under favourable conditions, undergoes a chemical reaction where product is ethyl alcoholalcohol.
Reaction equation – in words:
Reaction equation – molecular form:
The reaction equation shows that alcohol fermentationalcohol fermentation is a process that takes place under the influence of yeast. Its products are ethyl alcohol and carbon dioxide.
Before you conduct the experiment and/or watch the movie „Mixing ethanol with water”, write down the research question and hypotheses. Also note observations and conclusions from the study. Pay attention to what happens to the volume of the mixture.

Film dostępny na portalu epodreczniki.pl
Nagranie filmowe przedstawia eksperyment mieszania etanolu z wodą, ethanol with water. Do przeprowadzenia eksperymentu potrzebne są probówka z wodą (test tube with water), probówka z alkoholem etylowym (test tube with denatured alcohol), korek (plug), marker. Do probówki wypełnionej do połowy wodą należy wlać po ściance taką samą objętość alkoholu, uważając, aby podczas wlewania ciecze nie uległy wymieszaniu. Następnie na ścianie probówki zaznaczamy markerem poziom cieczy w probówce. Probówkę zamykamy korkiem i mieszamy dokładnie mieszaninę. Po wymieszaniu alkoholu z wodą, okazuje się, że mieszanina cieczy zmniejszyła swoją objętość w probówce. Wysokość cieczy w probówce znajduje się teraz poniżej zaznaczonej wcześniej markerem kreski.
Does ethanol dissolve in water?
Ethanol readily dissolves in water to form a homogeneous mixture.
two test tubes,
water,
ethanol (better effect will be obtained using denatured alcoholdenatured alcohol),
waterproof marker.
Measure out equal amounts of water and alcohol.
Gently pour alcohol into the test tube with water so that the liquids do not mix.
Mark the liquid level on the test tube with the marker.
Close the tube and shake it.
Mark the liquid level after mixing.
Compare the volume change of the liquid before and after mixing.
Ethyl alcohol slowly poured into a test tube with water creates a noticeable layer. The boundary between alcohol and water disappears after mixing the liquid. The resulting homogeneous mixture, whose components cannot be distinguished by means of sight. This mixture has a smaller volume than ingredients (water and ethanol) used to make it. The reason for this physical phenomenon, called contraction of volumecontraction of volume, is the interaction between the molecules of the mixture components.
The contraction phenomenon is taken into account in the spirits industry in the technological processes and in the calculation of production efficiency. When adding 50 litres of water to 50 litres of ethyl alcohol (grain alcohol), not 100 but 96.3 litres of solution are produced.
A mixture of ethanol and water with a 95.6% ethanol content is called grain alcohol.
Physical property | Methanol | Ethanol |
matter of state | liquid | liquid |
colour | colourless | colourless |
solubility in water | no limits | no limits |
boiling point | 64.7°C | 78.3°C |
density | 0.792 g/cmIndeks górny 33 | 0.789 g/cmIndeks górny 33 |
Chemical properties of methanol and ethanol
By examining methanol and ethanol, we will answer the following questions:
What are chemical properties of alcohols?
Is there any similarity in their behaviour during chemical reactions?
Does the presence of the functional group in alcohol molecules affect the pH of their aqueous solutions?
Smell
Both methanol and ethanol are characterized by a sharp, irritating odour. Testing these alcohols only with smell can lead to confusion.
pH of alcohols
Do aqueous solutions of alcohols undergo electrolytic dissociation? Is the pH of aqueous solutions of alcohols the same as the pH of bases, since they have a hydroxyl group similar to hydroxides? For testing the pH of aqueous solutions of alcohols, known indicators can be used: red cabbage brew, phenolphthalein solution, universal indication strip
Before you conduct the experiment and/or watch the movie „Testing the pH of aqueous ethanol solution”, write down the research question and hypotheses. Also note down observations and conclusions from the study. Pay attention to what happens to the indicators during the experiment.

Film dostępny na portalu epodreczniki.pl
Nagranie filmowe przedstawia badanie pH roztworu etanolu, pH of ethanol solution. Do przeprowadzenia badania potrzebne są probówka z fenoloftaleiną, phenolphtalein, probówka z wywarem z czerwonej kapusty, decoction of red cabbage, paski papierowe wskaźniki, paper indictaor, dwie probówki z etanolem, ethanol. Do jednej probówki z etanolem wlewamy wywar z czerwonej kapusty i mieszamy. Do drugiej probówki z etanolem wlewami fenoloftaleinę i mieszamy. Wskaźniki dodane do roztworów etanolu nie zmieniły koloru. Następnie badamy pH roztworów za pomocą uniwersalnego papierka wskaźnikowego, kolor papierka również nie ulega zmianie.
What is the pH of aqueous ethanol solution?
The alcohols contain group in their molecules, so the aqueous ethanol solution will be alkaline
three test tubes,
aqueous ethanol solution (grain alcohol),
red cabbage brew,
phenolphthalein solution,
universal indication strip.
Pour approximately 3 cmIndeks górny 33 of alcohol into three tubes.
Examine the smell and determine the solubility of alcohol in water.
Pour a few drops of a red cabbage brew into one tube and add one drop of phenolphthalein to the other one. Put the universal indicator stripe into the third test tube.
Answer what you have observed.
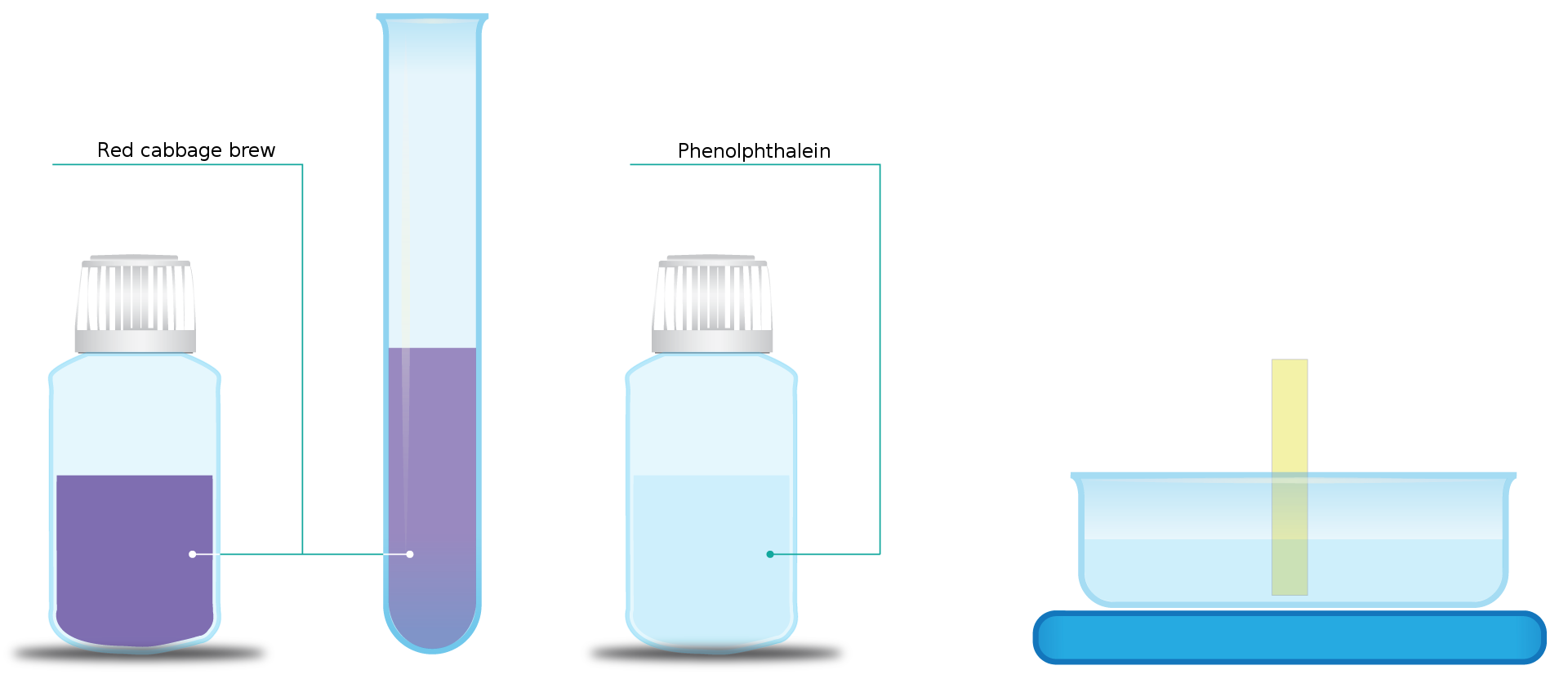
The indicators added to the ethanol solutions did not change the colour. The pH of aqueous solutions of alcohols is neutral. It means that there is no excess of HIndeks dolny 33OIndeks górny ++ ions and OHIndeks górny -- ions in an aqueous solution. Thus, the alcohols do not undergo ion dissociation.
Flammability test of ethanol
Ethanol is a flammable substance. With unrestricted oxygen access, a complete ethanol combustion occurs.
When the fire torch is moved closer, the alcohol vapour ignites. The alcohol burns with a bluish flame. The products of the total combustion of ethanol are carbon dioxide and water:
Complete methanol combustion
The products of the total combustion of methanol are carbon dioxide and water:
Incomplete combustion of alcohols
Incomplete combustion takes place under conditions of limited oxygen supply.
Select properties for ethanol.
- It is flammable, boiling point of 64.7oC.
- Mix with water without restrictions.
- It is alkaline due to the presence of hydroxyl group.
- Has a characteristic, sharp smell.
- Burns blue, yellow and smoky flame.
- It is a white liquid.
- Has a sharp taste.
Summary
Methanol and ethanol have many common physical properties and are therefore difficult to distinguish.
Methanol is a poison.
Only methanol, ethanol and propanol are well soluble in water, each subsequent alcohol in the homologous series already less.
The pH of aqueous solutions of alcohols is neutral.
Methanol and ethanol are flammable substances. These are subject to complete and incomplete combustion reactions.
Write down the equation of the reaction of butyl alcohol combustion, if the reaction product is a gas causing clouding of limewater.
Key words
alcohol, hydrocarbons, monohydric alcohols, methanol, ethanol, methyl alcohol, ethyl alcohol, volume contraction
Glossary
alkohole – pochodne węglowodorów, w których co najmniej jeden atom wodoru zastąpiono grupą hydroksylową
fermentacja alkoholowa – przemiana glukozy pod wpływem enzymów wytwarzanych przez drożdże; jej produktami są alkohol etylowy i tlenek węgla(IV)
denaturat – (spirytus skażony) – alkohol etylowy z dodatkiem substancji o przykrym zapachu i smaku, nienadający się do spożycia; zabarwiony na fioletowo lub czerwono; nie wolno spożywać denaturatu ani innego rodzaju skażonego alkoholu
kontrakcja objętości – zjawisko fizyczne, które polega na zmianie objętości roztworu podczas mieszania jego składników
spirytus – wodny roztwór alkoholu etylowego o stężeniu około 96%
spirytus drzewny – zwyczajowa nazwa otrzymywanego w wyniku suchej destylacji drewna roztworu alkoholu metylowego