Calculation of concentration and solubility
that the solubility in water is expressed as the maximum number of grams of substance that is dissolved in 100 g of water at a given temperature and pressure (the pressure is taken into account in the case of gas solutions, e.g. oxygen in water, and when the solute is solid or liquids, the pressure is of little importance and can be omitted);
that the graph showing the dependence of the solubility of a given substance on the temperature is called the solubility curve;
that the percentage determines the number of grams of solute contained in 100 grams of solution and can be calculated from the formula: .
to calculate the percentage concentration of a saturated solution at a given temperature using a solubility chart
to determine the solution solubility based on the percentage of saturated solution at a given temperature
How we calculate the percentage of saturated solution?
The solution saturated at a given temperature contains a defined amount of solute in a given mass of solvent. On the basis of these values, the percentage concentration of the solution can be calculated.
Size | Solubility | Weight of the solution | Percent concentration |
importance | the maximum mass of substance that can be dissolved in 100 g of water at a given temperature |
Calculate the percentage of the saturated copper(II) sulphate solution at 80°C.
Which saturated solution has a higher percentage: sodium chloride at 50°C or sodium nitrate at 10°C? Make the right calculations that will answer this question.
The more mass of solute falls on a specific mass solventsolvent, the percentage concentration of the solution is higher. Due to the fact that the solubility of solids usually increases with temperature, the concentration of the saturated solution of these substances also increases.
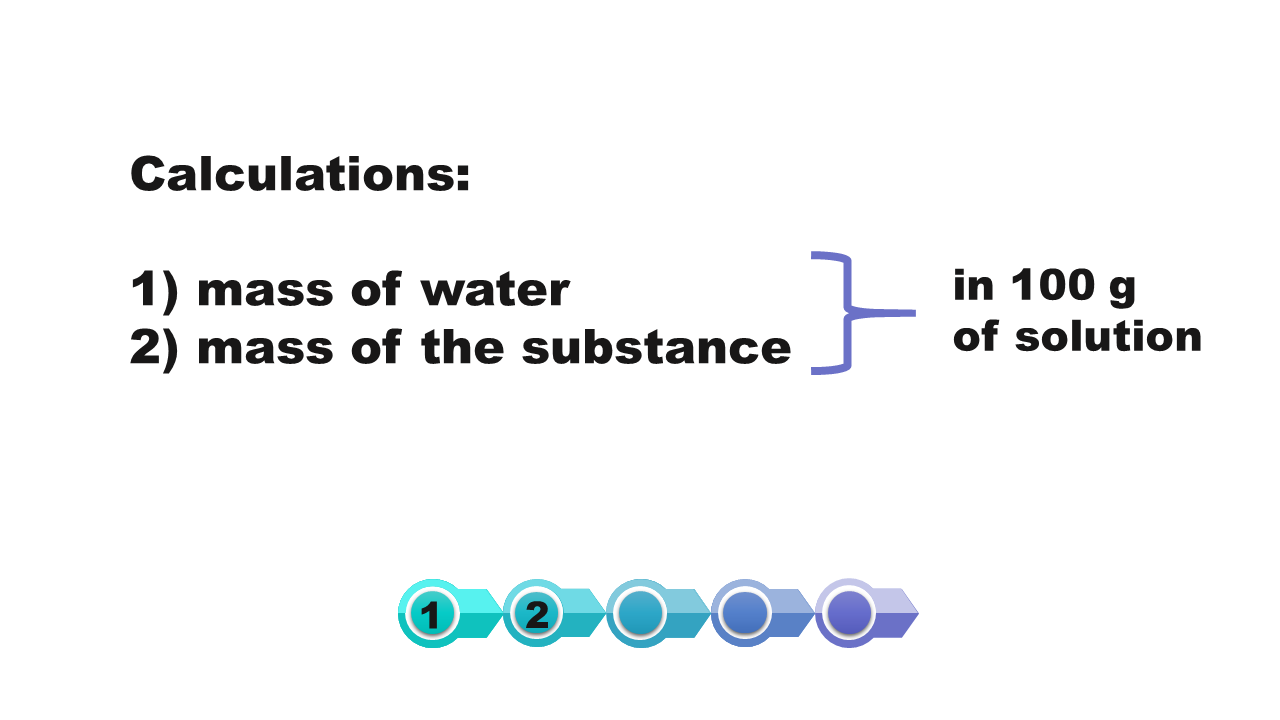
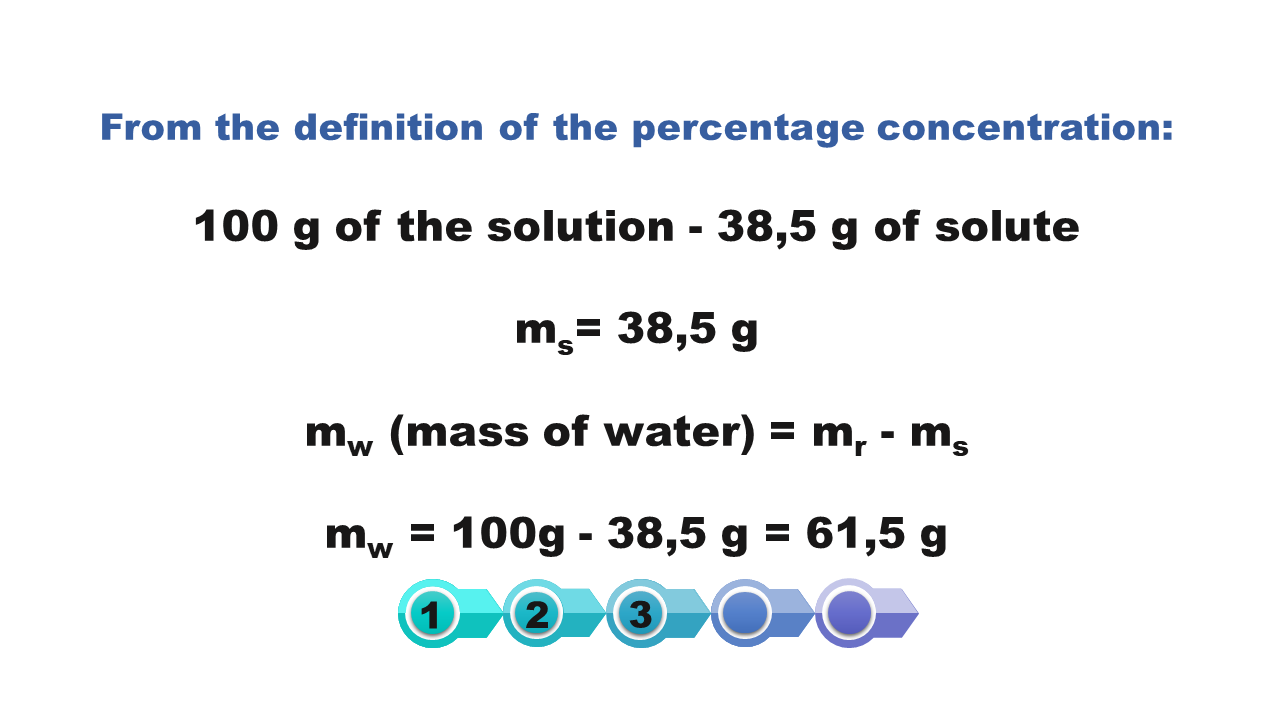
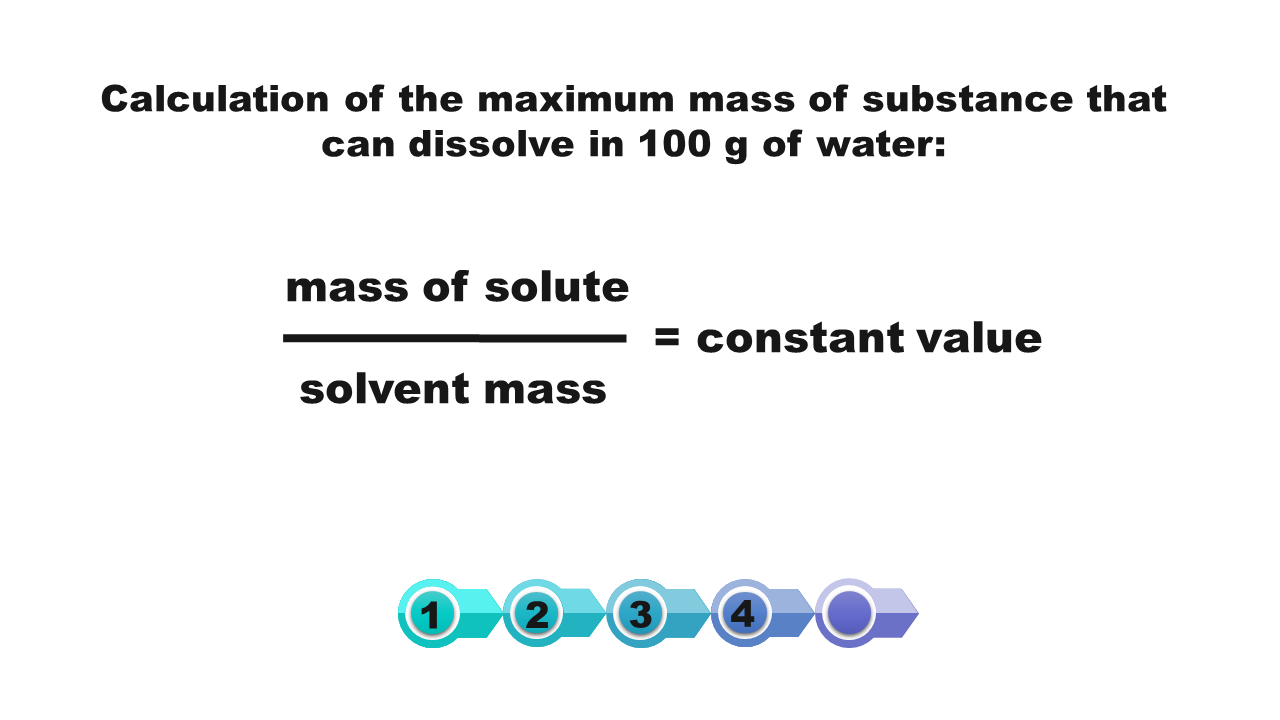
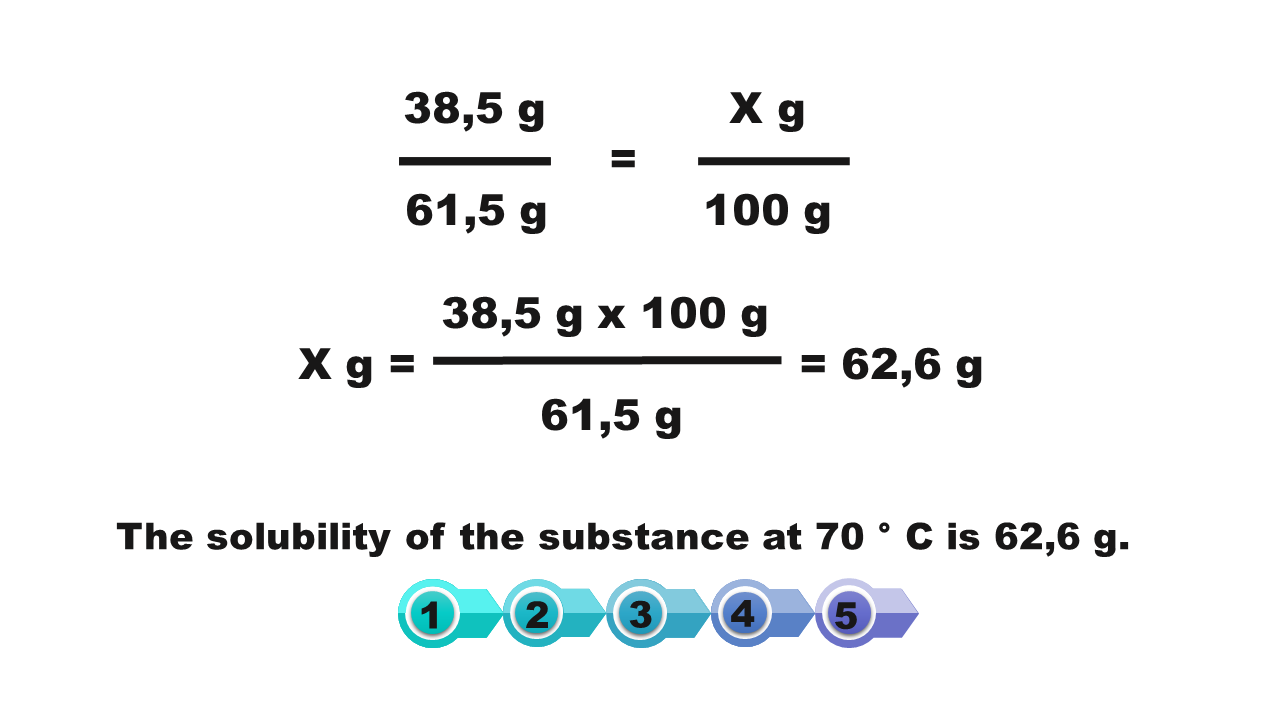
How we calculate the solubility of a substance in a saturated solution with a known percentage concentration?
If at a given temperature we know the percentage of saturated solution of a substance, then we can calculate the solubility of this substance at a certain temperature.
Before solving tasks, watch the presentation, it will guide you through the process of solving the task.
Calculate the solubility of the substance at 50°C, if the solution saturated at this temperature has a concentration of 53.27%.
Preparing a solution of potassium nitrate in water, the student noticed that at a temperature of 60°C, a small amount of crystals remained at the bottom of the vessel. What should I do to let these crystals dissolve? Highlight the right answers:
- pour off some of the solution
- top up with water
- heat the solution
- evaporate a small amount of water.
Create a multiple-choice test based on today's lesson. Then exchange your questions with a friend or classmate.
Question: ...
- ...
- ...
- ...
- ...
Summary
Based on the knowledge of the solubility of the substance, the percentage of saturated solution can be calculated.
Knowing the percentage concentration of a saturated solution of a substance, you can calculate its solubility.
Keywords
percentage concentration of the solution, solvent
Glossary
stężenie procentowe roztworu – jest to liczba gramów substancji zawarta w 100 gramach roztworu, wyrażona w procentach
rozpuszczalnik – ciecz zdolna do tworzenia roztworu po zmieszaniu z ciałem stałym, inną cieczą lub gazem