Chemical structure of organisms
the matter is composed of different substances, e.g. water, salt, iron;
substances are composed of particles which give them their characteristic features;
organisms take up substances from the environment and process them.
to give examples of elements that build organisms;
to explain why carbon and water are so important for life;
to show a link between the chemical structure and properties of carbohydrates, proteins, fats and nucleic acids and their biological function.
Nagranie dostępne na portalu epodreczniki.pl
Nagranie dźwiękowe dotyczące chemicznej budowy organizmów
Elements that build organisms
All substances are made up of atoms. If the substance consists of identical atomsatoms, which are not permanently bonded to each other, it is called a chemical elementchemical element.
Let us trace the content of some elements in the human body. On average, one kilogram of the human body contains 650 g of oxygen, 180 g of carbon, 100 g of hydrogen and 30 g of nitrogen. These four elements are the basic components of living matter. If we add further elements to them: phosphorus (10 g) and sulphur (3 g) – we obtain a group of so‑called biogenic elementsbiogenic elements. Without them, life on Earth would not exist.
Further elements in the sample analysed are 15 g of calcium, 4 g of potassium, 2 g of sodium, 1 g of magnesium and 1 g of chlorine. Together with the biogenic elements, they are called macroelementsmacroelements, because their amount in organisms is large enough to be easily determined by weight. The remaining elements are the so‑called microelementsmicroelements, i.e. elements occurring in trace amounts, each of which constitutes less than 0.01% of dry matterdry matter. They are as important for the development and functioning of organisms as macroelements. These include, among others, iron, iodine, copper, zinc, fluorine and silicon.
Name and chemical symbol of the element | Biological significance | |
MACROELEMENTS | ||
BIOGENIC ELEMENTS | Carbon C | the primary component of all organic compounds |
determines life on Earth | ||
Hydrogen H | is part of all organic compoundsorganic compounds | |
component of water | ||
Oxygen O | is part of all organic compounds (except for hydrocarbonshydrocarbons) | |
one of the components of the Earth’s atmosphere | ||
necessary for breathing for most organisms | ||
Nitrogen N | component of proteins and nucleic acids | |
Sulphur S | is part of many proteins and some carbohydrates | |
participates in respiratory processes in the cell | ||
Phosphorous P | component of nucleic acids | |
builds bones and teeth | ||
component of fluids filling the interior of organisms | ||
Calcium Ca | component of bones, mollusc shells and plant cell walls | |
essential in the blood clotting and muscle cell contraction processes | ||
Potassium K and Sodium Na | responsible for cellular hydration | |
take part in the conduction of nerve impulses | ||
Magnesium Mg | regulates the functioning of the nervous system | |
necessary for photosynthesis and respiration processes | ||
component of green pigment of plants – chlorophyll | ||
Chlorine Cl | forms hydrochloric acid – component of gastric juice | |
MICROELEMENTS | ||
Iron Fe | component of haemoglobin – red pigment of blood | |
Copper Cu | takes part in cellular respiration | |
component of enzymes involved in the formation of chlorophyll and haemoglobin | ||
Zinc Zn | necessary for the growth and development of plants | |
takes part in wound healing | ||
Fluorine F | component of bones and tooth enamel |
Answer the question: What influence do the elements contained in mineral water have on cress development?
Supplying cress with mineral water causes rapid growth of plants.
cress seeds,
2 plates,
cotton wool,
mineral water of known elemental composition,
distilled (demineralised) water.
Place a thick layer of cotton wool on the plates moistened with distilled water (Sample 1) and mineral water (Sample 2).
Sow 30 seeds each on the plates and place them in a sunny place with a constant temperature of about 20°C.
If necessary, water the plant raising with distilled water (Sample 1) and mineral water (Sample 2).
Take measurements every day for 14 days and compare the height of the germinated plants.
Download the attachment. Write down the results of the experiment and the conclusion.
If a cress watered with mineral water grows better than a cress supplied only with distilled water, it means that the elements contained in the mineral water are probably conducive to its faster development.
Place a drop of distilled water and a drop of mineral water next to it on a clean, dark plate. Allow the water to dry. Explain why there is only one drop mark left on the plate. OR Give examples of elements that build organisms;
Chemical compounds present in organisms
Organisms are composed of the same basic chemical compoundschemical compounds, but in different proportions. The human body consists on average of 65% of water and 2% of other inorganic compoundsinorganic compounds. The rest, i.e. about 33%, are molecules of organic compounds composed of numerous elements, the most important of which is carbon. Carbon is distinguished by its ability to form long chains of various shapes. They constitute the “backbone” to which other elements can be added. This results in creation of complex molecules of compounds such as proteinsproteins, carbohydratescarbohydrates, fatsfats and nucleic acidsnucleic acids. They build bodies of organisms, are a source of energy for their functioning and regulate processes occurring in them. Organic compounds also include vitamins responsible for the proper course of life processes.
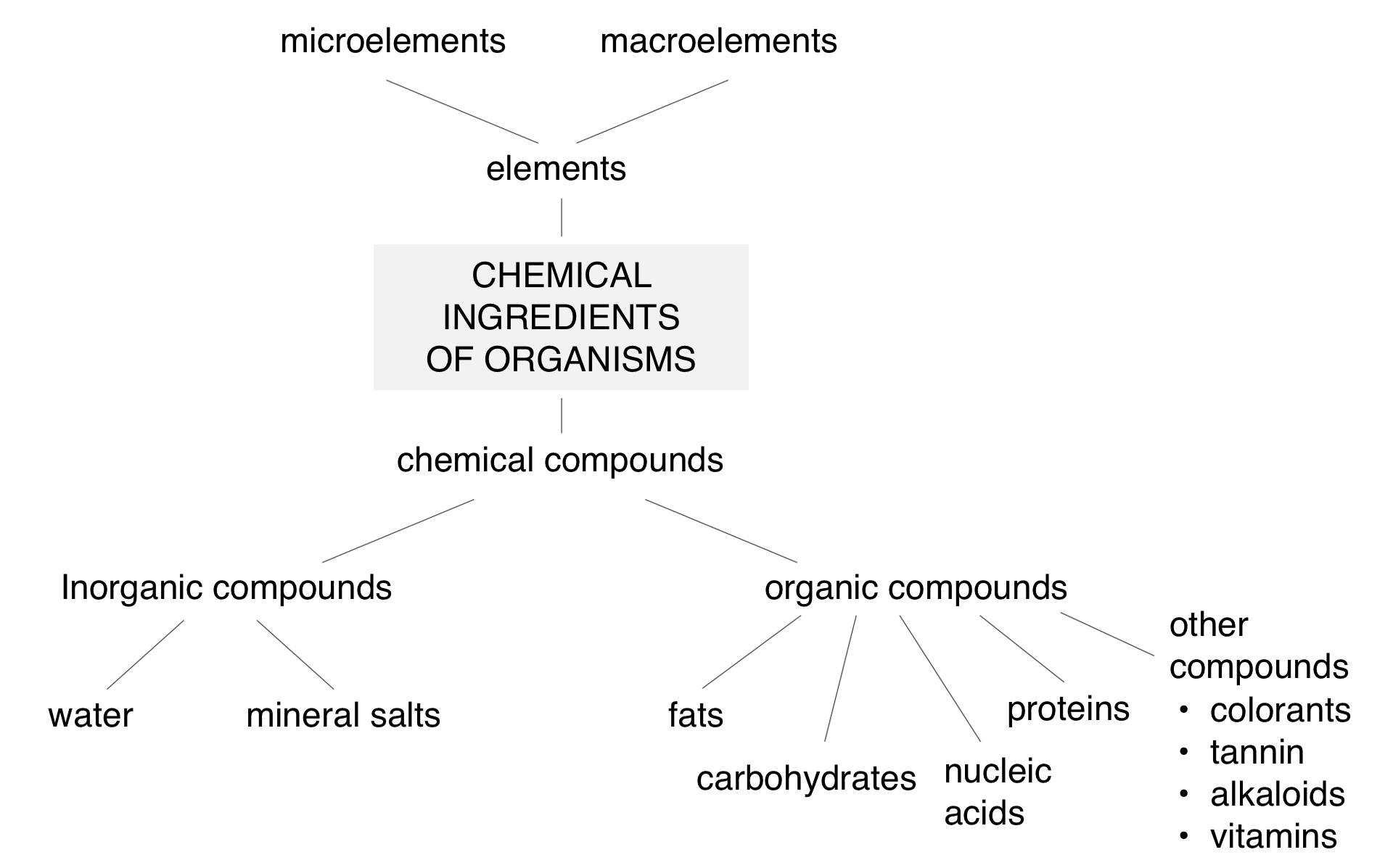
Chemical composition of organisms (in%):
Components | Plants | Animals |
Water | 75 | 60 |
Mineral compounds | 2 | 4 |
Carbohydrates | 18 | 5.8 |
Fats | 0.5 | 11 |
Proteins | 4 | 19 |
Nucleic acids | 0.5 | 0.2 |
Biological function of water
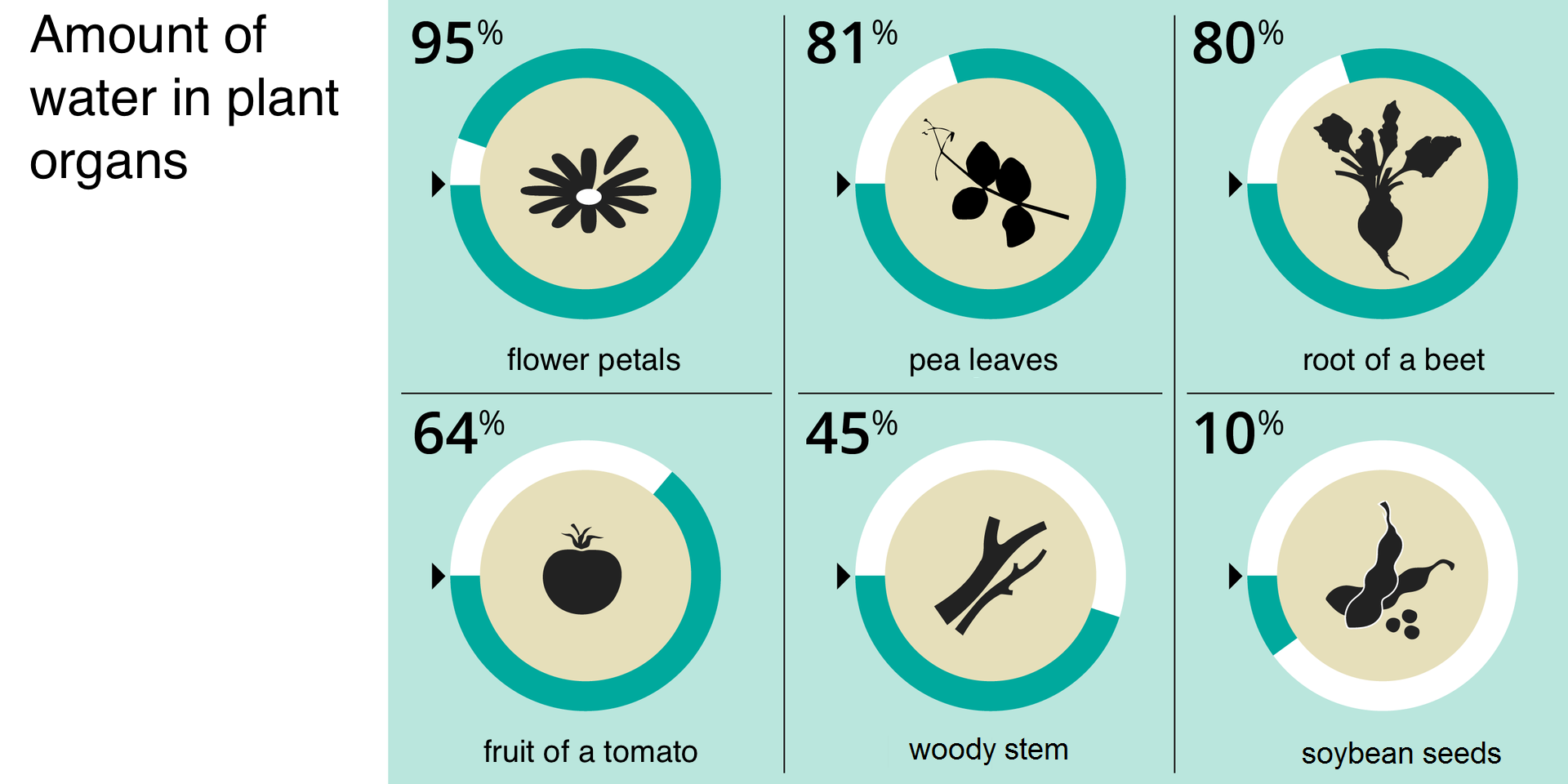
WaterWater is one of the most common substances on Earth. Without it, life would not exist. It is found in cells and tissue fluids that fill the intercellular spaces and in body fluids (e.g. blood) of animals. It is also found in plant tissues and organs. The average water content in organisms is not the same, it depends on the species and living environment, on the age and condition of the organism, as well as on the type of organ. The chemical structure of a water molecule determines its physical properties, and these affect its biological functions. Water is the environment in which most life processes take place and is a solvent for many chemical compounds. In addition, it enables regulation of the body temperature and influences the size and shape of the cells. The embryos of animals develop in an aquatic environment that provides them with a constant temperature and humidity.
Explain which physical properties of water allow your body to function.
Determination of the water content in leaves. OR What is water content in leaves?
a few fresh leaves of different plants,
burner or candlestick,
heat‑resistant test tubes,
test tube holder,
laboratory scales.
Weigh all leaves separately.
Put each leaf into a different test tube and heat it over the burner until the water evaporates completely.
Weigh the dried leaves.
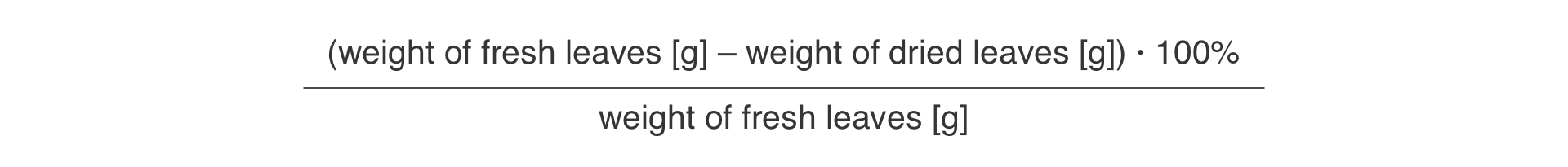
Calculate the percentage content of water in each leaf.
Open and fill in the attachment.
Use the following formula to calculate the percentage of water in the leaves:
Plant leaves differ in their water content.
Decide whether you can, based on the results of the observation 1., draw the following conclusion: “The water content of the leaves depends on the plant species”. Justify your answer. OR Why the water content of the leaves depends on the plant species?
Complete the text with the words given below.
smallest, element, atoms, carbon
An element is made up of ................. An atom is the ................ part of an ................. Iron is an element, as is also .................
Complete the last sentence.
The following objects are made up of atoms of one kind: a ring is made up of gold atoms, a teaspoon is made up of aluminium atoms and a brooch is made up of silver atoms.
Gold, aluminium, silver are .................
The chemical compound is made up of
- atoms
- particles
- elements
- molecules
Nitrogen, oxygen, carbon dioxide and water are the components of the air. The air is
- a chemical compound
- an element
- a particle
- s substance
Mark the words that have been used correctly in the sentences.
green
Protein is a chemical compound and is made up of atoms. Water particle consists of two oxygen elements and one hydrogen atom. Carbon dioxide is a chemical compound consisting of two elements: water and carbon. Oxygen is a substance composed of identical atoms. Glucose building elements are carbon, oxygen and water.
Indicate the correct ending of the sentence. Our bones contain
- mineral salts
- calcium element
- magnesium chemical compounds
- atoms of different elements
Which chemical component prevails in the organism?
- calcium
- nitrogen
- water
- glucose
Complete the sentences.
slowly, steaming, freezing winter, freeze, cools down, water, 4, accumulates, evaporation, temperature
During the .............................., water with temperature of .............................. Celsius degrees .............................. at the bottom of the tank. Fish swims close to the bottom and therefore does not ...............................
Water absorbs large amounts of heat during ............................... This is how it .............................. its surroundings. The main component of sweat is ............................... The .............................. sweat cools down our body.
The water heats up .............................. and loses heat slowly. Thanks to this, the .............................. in the tanks does not change rapidly.
A chemical compound is
- magnesium salt
- carbon dioxide
- nitrogen
- carbon
Water is a good solvent, therefore
- regulates the functioning of the organism
- cools down the body by evaporating
- transports chemical compounds
- collects large amounts of energy
Conclusion
Among the elements building organisms, we can distinguish macroelements and microelements.
The elements without them life would not exist are carbon, hydrogen, nitrogen, oxygen, phosphorus and sulphur.
Carbon is the main component of organic compounds.
The specific properties of water make it an essential component of all organisms.
Proteins, fats, carbohydrates, nucleic acids are the basic organic compounds of the organisms.
Glucose is the main source of energy in cells.
Polysaccharides perform building and reserve functions.
Proteins are the basic building material of cells and regulate the course of life processes of organisms.
Fats are a source of energy and a reserve material for plants and animals.
DNA is a carrier of genetic information.
2. Explain the importance of water as the external environment of the fish and as a substance in the cell of the fish's body.
Keywords
inorganic compounds, atom, element, particle
Glossary
atom – najmniejsza, niepodzielna część pierwiastka
białka – wielkocząsteczkowe związki organiczne zbudowane z aminokwasów; występują we wszystkich organizmach; pełnią funkcje budulcowe, enzymatyczne, transportujące, odpornościowe i regulacyjne
celuloza – wielocukier nierozpuszczalny w wodzie; buduje ściany komórkowe roślin
chityna – wielocukier nierozpuszczalny w wodzie; buduje ściany komórkowe grzybów i szkielety zewnętrzne stawonogów np. owadów
glukoza – cukier prosty; podstawowe źródło energii dla organizmów
glikogen – wielocukier; stanowi materiał zapasowy u zwierząt (występuje głównie w ich wątrobie i mięśniach)
kwasy nukleinowe – organiczne związki chemiczne; przechowują informację genetyczną organizmu i pośredniczą w produkcji białek; znane są dwa podstawowe typy naturalnych kwasów nukleinowych: kwasy deoksyrybonukleinowe (DNA) i kwasy rybonukleinowe (RNA)
makroelementy – pierwiastki, z których każdy stanowi nie mniej niż 0,01% suchej masy organizmu; oprócz pierwiastków biogennych należą do nich: magnez (Mg), wapń (Ca), potas (K), sód (Na) i chlor (Cl); są niezbędne do prawidłowego wzrostu i rozwoju organizmów
mikroelementy – pierwiastki, których ilość nie przekracza 0,01% suchej masy organizmu; są niezbędne do prawidłowego rozwoju organizmu, a ich brak lub niedobór może powodować zaburzenia funkcjonowania i choroby; należą do nich m.in. żelazo (Fe), jod (I), miedź (Cu), cynk (Zn), fluor (F), krzem (Si)
pierwiastek chemiczny – substancja złożona z jednakowych atomów
pierwiastki biogenne – pierwiastki stanowiące podstawowy składnik związków organicznych; są to: węgiel (C), wodór (H), tlen (O), siarka (S), azot (N), fosfor (P)
skrobia – wielocukier zbudowany z cząsteczek glukozy, nierozpuszczalny w wodzie; materiał zapasowy roślin
sucha masa – masa substancji po odparowaniu z niej wody
tłuszcze – związki organiczne; stanowią substancje zapasowe roślin i zwierząt oraz materiał ochronny i termoizolacyjny u zwierząt
węglowodany – inaczej cukry; organiczne związki chemiczne złożone z atomów węgla oraz wodoru i tlenu; ze względu na budowę dzielone na cukry proste, dwucukry i wielocukry; jedna z podstawowych grup związków chemicznych wytwarzanych przez organizmy
węglowodory – organiczne związki węgla i wodoru występujące w ropie naftowej
woda – jedna z najczęściej spotykanych substancji na Ziemi; stanowi średnio 2/3 masy organizmów; doskonały rozpuszczalnik związków organicznych; uczestniczy w przebiegu większości reakcji chemicznych w komórkach; transportuje produkty przemiany materii, substancje odżywcze, enzymy; reguluje temperaturę organizmów; stanowi płynne środowisko wielu reakcji biochemicznych
związek chemiczny – jednorodna substancja złożona z wybranych atomów trwale połączonych w cząsteczki
związek nieorganiczny – związek, który nie zawiera atomów węgla; wyjątkiem są zawierające węgiel tlenki węgla, kwas węglowy i węglany
związek organiczny – związek chemiczny, w skład którego wchodzą atomy węgla, często tworzące długie łańcuchy, a także atomy innych pierwiastków; wyjątkiem są zawierające węgiel tlenki węgla, kwas węglowy i węglany; związki organiczne powstają głównie w organizmach