Density of substances and their mixtures in everyday life: density
what is mass;
that a body of the same mass, depending on the force of gravity and the place of measurement, may have different weights;
what is the volume;
which is the unit of volume in the SI system;
how to indicate ways to determine the volume of a substance.
explain on the basis of the internal structure why the density of solids is greater than the density of gases;
carry out calculations regarding mass, density and volume.
What the density is?
DensityDensity is the relation (ratio) between the given mass of substance and the volume it occupies - a physical characteristic of a given substance.
Look at the drawing and compare the size and weight of the volleyball and the bowling ball. Consider the ratio between mass and volume and the internal structure of these objects.

The bowling ball and the volleyball are the same size, but the first one has a higher mass, because it is made of plastic, which contains many tightly packed elementary particles. The volleyball is filled with air containing fewer elementary particles that are more apart. Typically, solids have the highest density, liquids – lower, and gases – the lowest.
The density of a given substance which has a solid state of matter is determined by modelling - making a cube with an edge of 1 m and then weighing it. The mass of such a cube expressed in kilograms is numerically equal to the density of the substance.
How to calculate the density?
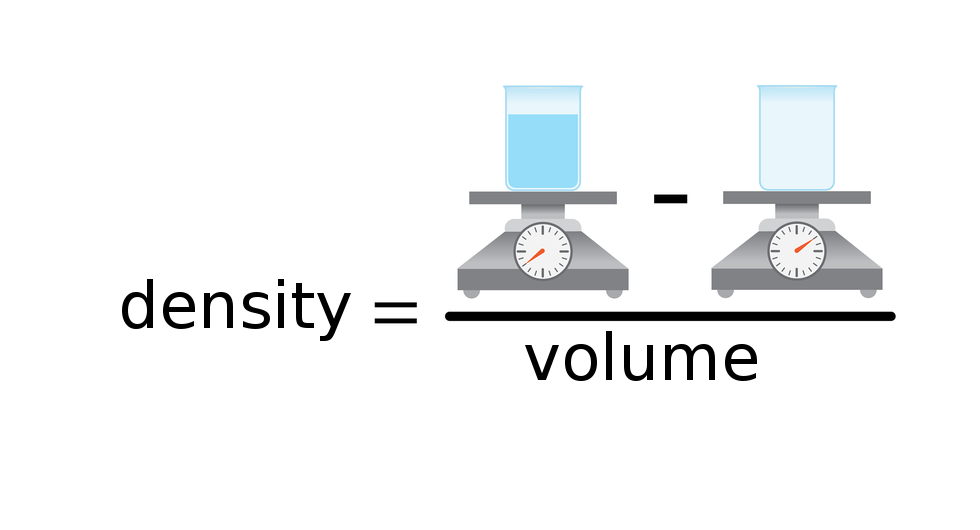
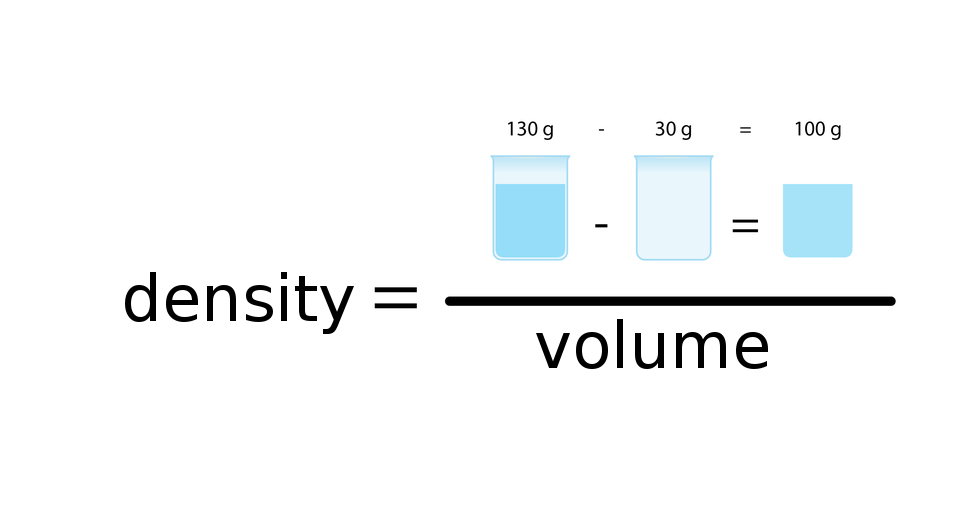
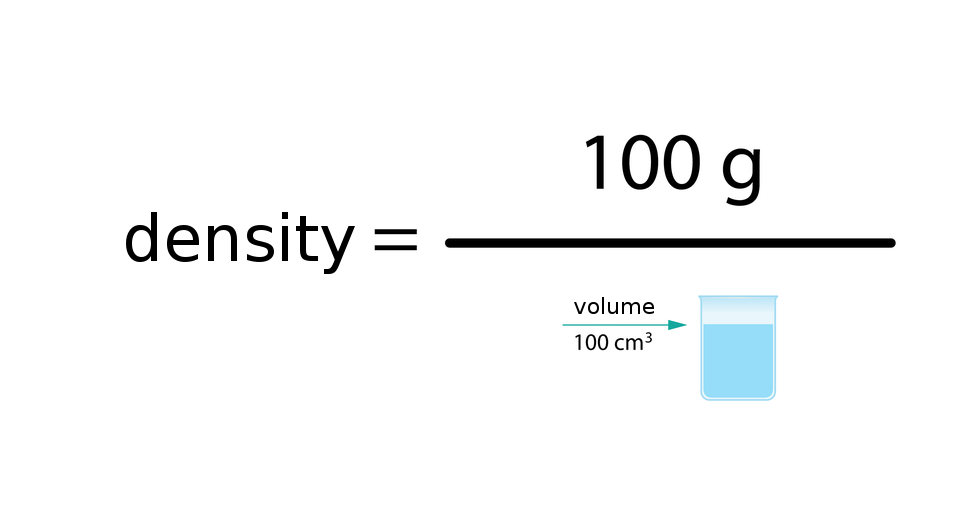
DensityDensity () is the ratio between mass () of the substance and the volume () that given mass occupies. It is expressed by the formula:
The usual unit of the solids and liquids density is , and the usual unit of gases density is . Chemists usually mark density with a symbol (English density), and physicist mark it with Greek letter (ro).
The density of substance depends on:
temperature – it usually decreases with increasing temperature;
pressure – just for gases, because the impact of pressure on liquids and solids is so small that we omit it.
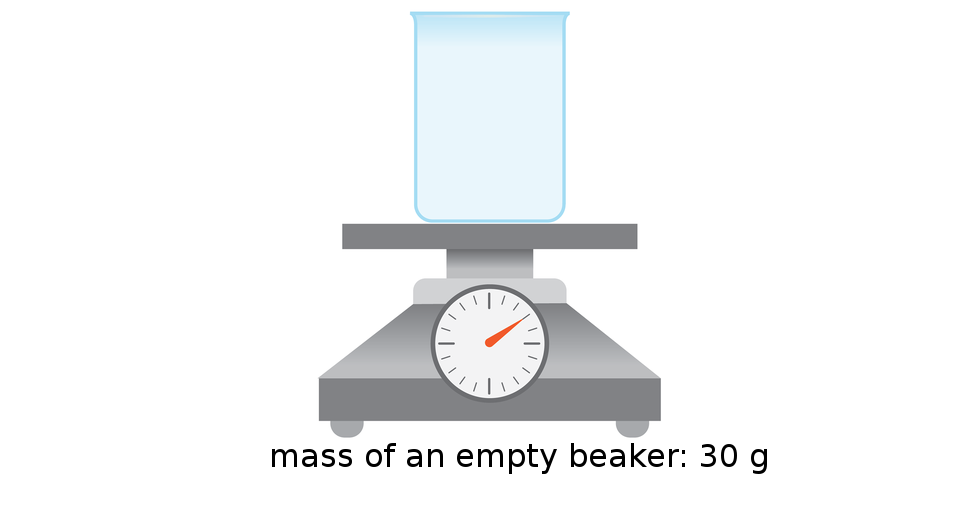
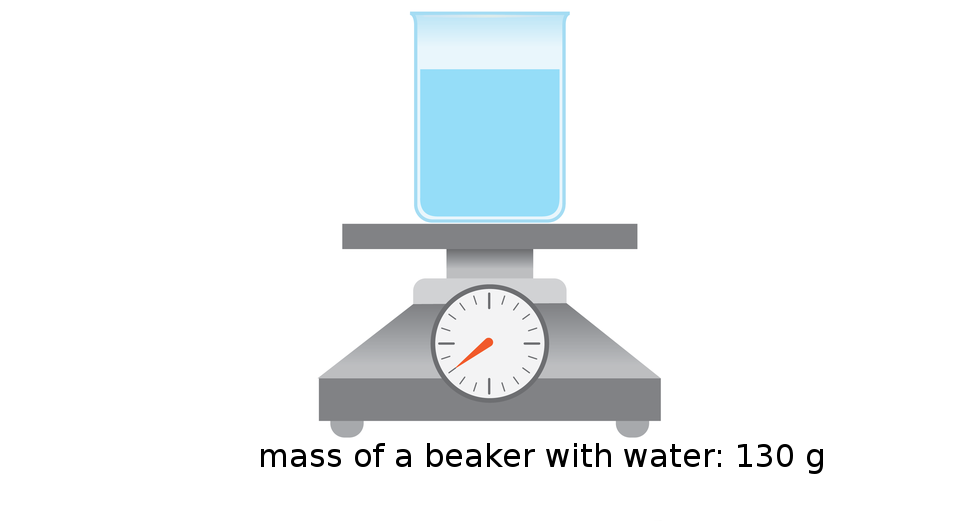
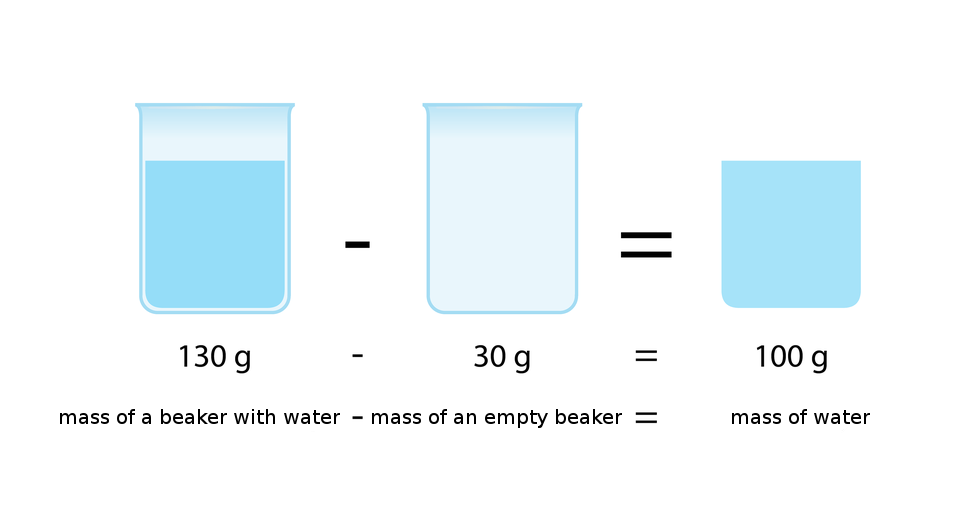
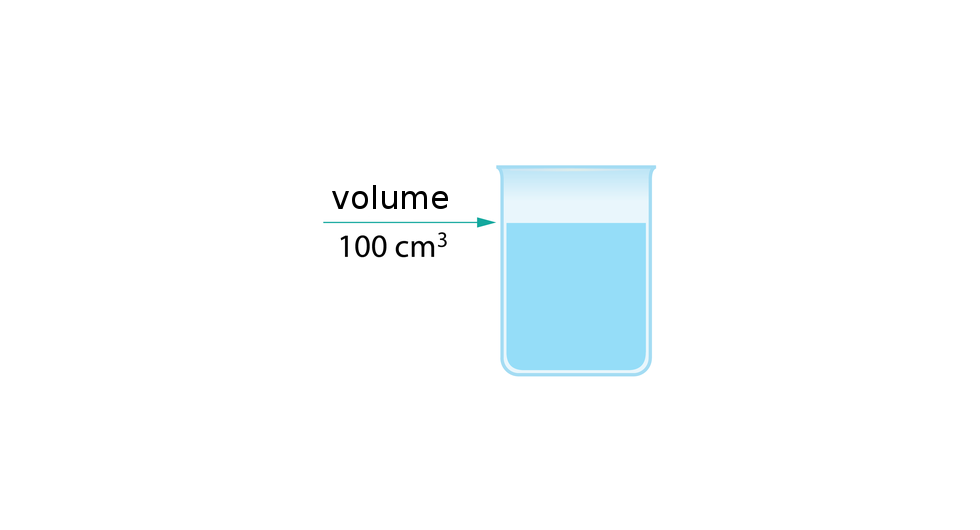
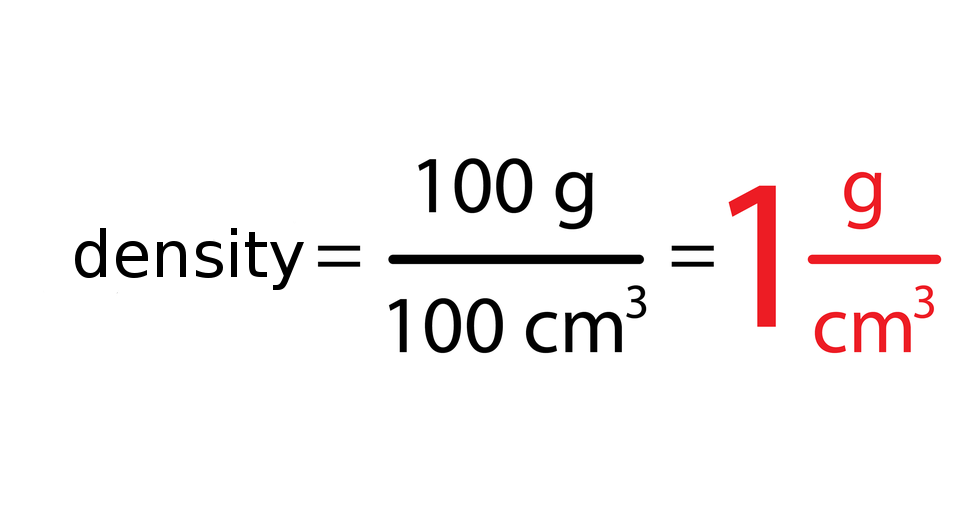
The density of substance is often compared with the density of water. How much does a litre of water weigh? View the gallery of drawings and memorise how the mass depends on volume and how the water density is calculated.
Before the movie “Water Rainbow” formulate a research question and hypotheses. During the screening observe what happens with the liquids in beakers. What does it mean?

Film dostępny na portalu epodreczniki.pl
Nagranie video abstraktu przedstawiające eksperyment "Water Rainbow". Na filmie widać sześć naczyń szklanych najpierw wypełnianych woda, potem do każdego naczynia dosypywany jest chlorek sodu (sodium chloride). Po wymieszaniu, do każdego naczynia dodawany jest barwnik spożywczy (food colouring) o innym kolorze. Następnie naukowiec nabiera pipetą (transparent straw) po trochu cieczy z każdego naczynia. Mimo iż nabrana woda jest w jednej pipecie wyraźnie widać wszystkie kolory osobno.
How do liquids of different densities behave?
Select one of the presented hypotheses, and then verify it.
Liquids with higher density float on the surface.
Liquids with higher density gravitate to the bottom.
rock salt,
water,
teaspoon or burette,
food colouring or dyes,
transparent straw,
6 beakers or high glasses (can be plastic cups).
Add respectively 1 to 6 teaspoons of salt to six beakers.
Pour approximately 125 cmIndeks górny 33 of warm water (half of glass) into each of these glasses and mix until the salt is completely dissolved.
Dissolve the dyes in water so that a different colour of the solution is obtained in each glass.
Cover the end of the straw with the thumb.
Holding the straw covered with your thumb, immerse the other end of the straw in a glass of water containing one teaspoon of salt. For a moment, move your thumb away from the straw outlet, draw up some water and quickly clog the straw again.
Proceed similarly with the remaining samples, sequentially drawing up the solutions into the straw, until six layers are obtained.
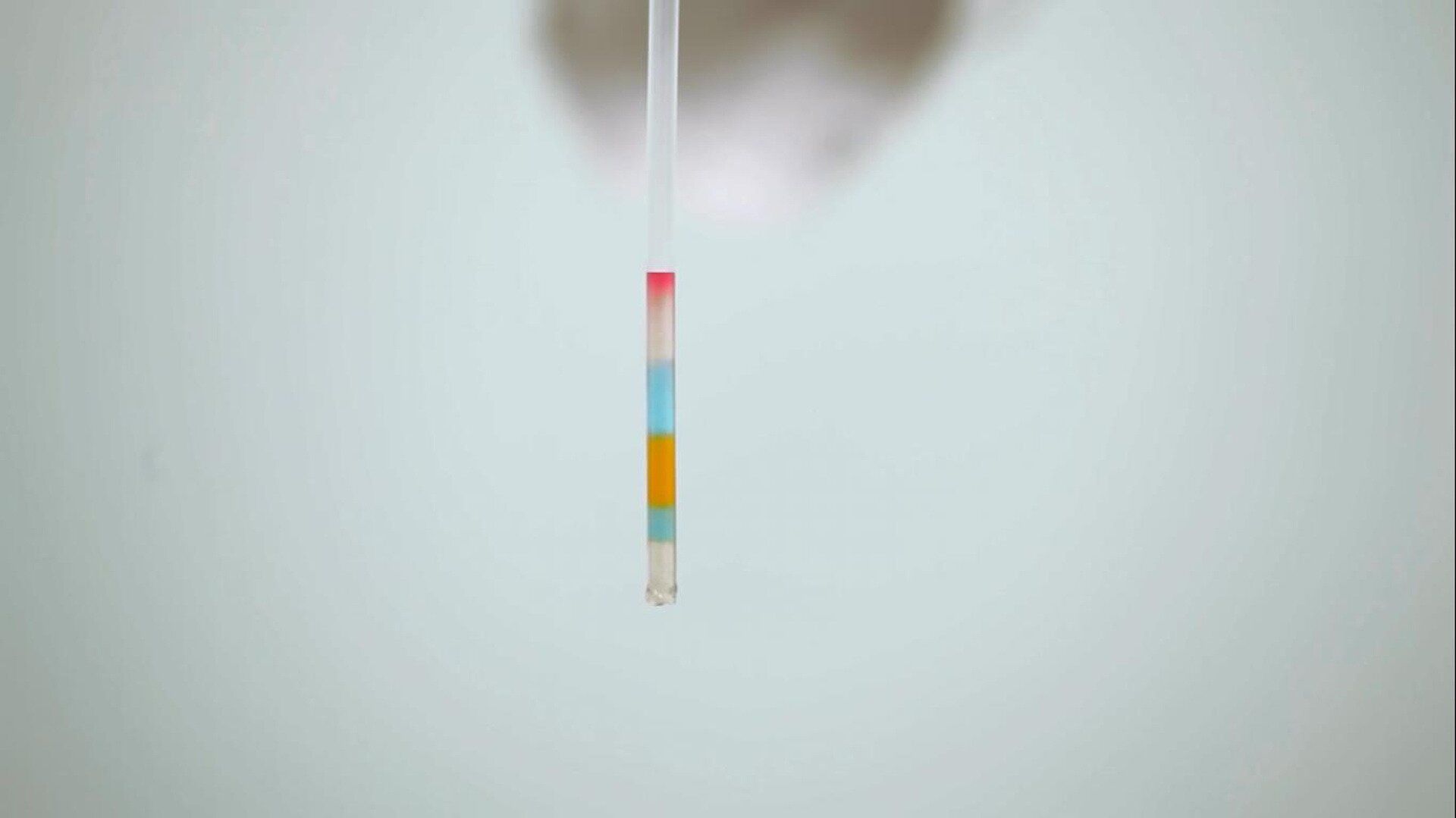
Consider:
What could happen if you use sugar instead of salt for the experiment?
What could happen if you immerse straw in the liquids in different order?
Before conducting the experiment “Drink Density” formulate a research question and hypothesis.
How can we compare the density of drinks?
Classic coke drink has a lower density than diet coke drink?
Classic coke drink has a higher density than diet coke drink?
- classic coke drink,
diet coke drink,
aquarium or a tall vessel,
water,
sugar,
scale.
You can calculate the density of the drink, if you know the mass and the volume. By placing beverage cans in a container filled with water, you can compare the cola density after considering the density of the cans themselves.
1. Weigh the cans of classic coke and diet coke. Remember to subtract the weight of an empty can from the weight of the canned drink. 2. Check the volume on the can or read it after pouring the content of the can to the measuring cylinder.
3. Calculate the density of the drink using the formula:
4. Compare the contents of both drinks. Think about what can have the biggest impact on density differences. You can compare their density with the density of water. Density of water is 1 .
Unit of substance density is:
- g/cm3
- kg
- dm3
- cm3/g
Summary
One of the physical properties of the substance is density.
Density is the size that characterizes a substance equal to the quotient of the mass and volume of a given substance.
Experimentally determining the density of the object, we can determine from which substance it was made.
The density of the substance depends on the temperature - it generally decreases with increasing temperature.
Keywords
density, substance
Glossary
właściwość fizyczna określająca masę 1 mIndeks górny 33 lub 1 cmIndeks górny 33 substancji, wyraża się w jednostkachlub