Distillation of crude oil
the structure and properties of hydrocarbons;
the natural sources of hydrocarbons;
the difference between complete and incomplete combustion of organic compounds.
to explain what crude oil distillation is;
to list groups of products formed during distillation of crude oil classified by their state of aggregation;
to list products formed during distillation of crude oil depending on process temperature;
to list and explain the use of crude oil distillation products;
to explain the difference between distillation of crude oil and hard coal.
Black gold – crude oil
Crude oil was mined already in antiquity. It was then used, without any processing, as a cure for skin diseases and rheumatism. It was also used for embalming corpses. Often crude oil was used for military purposes, for example flaming arrows and the so‑called Greek fire (a flammable liquid containing, among others, crude oil, tar, sulphur, saltpetre, rock salt, resin, quicklime) were used in combat. Crude oil, also known as petroleum, was probably formed as a result of the decomposition of plant and animal remains under anaerobic conditions, with participation of anaerobic bacteria. It is a mixture of several thousand (solid, liquid and gaseous) chemical substances, mainly hydrocarbons. It contains also sulphur, nitrogen and oxygen compounds. It looks like a dark brown dense liquid with pungent odour. It burns with a yellow, smoking flame.
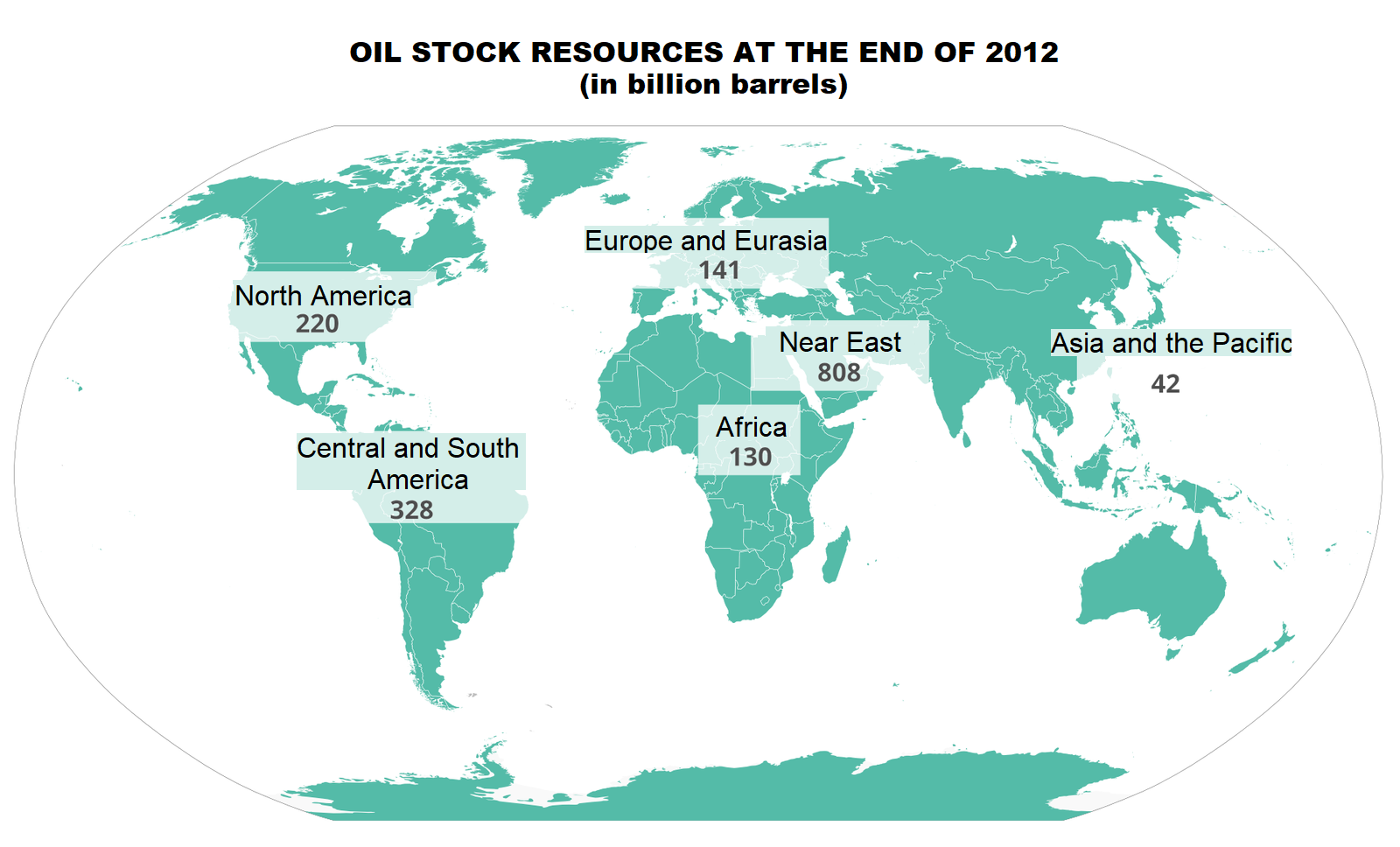
1. oil reserves at the end of 2012 (in billions of barrels)
2. North America (220)
3. Central and South America (328)
4. Europe and Eurasia (141)
5. Africa (130)
6. Middle east (808)
7. Asia and Pacific (42)
Crude oil processing began in Poland. In 1853 – Ignacy Łukasiewicz – Polish chemist, pharmacist and entrepreneur together with Jan Zeh developed a method for distillation of crude oil (fractional distillationfractional distillation, also known as rectification) and obtained in this way several products, such as kerosene. Łukasiewicz constructed a kerosene lamp in the same year. This discovery had a direct impact on the development of the oil industry. The oil mine he founded in Bóbrka was the first facility of this type in the world.
DistillationDistillation of crude oil
Formulate a research question and hypotheses before watching a video and carrying out the experiment. Write down your observations and conclusions.

Film dostępny na portalu epodreczniki.pl
Film z eksperymentem. Zmontowano zestaw do destylacji, a następnie w kolbie okrągłodennej umieszczono ropę naftową oraz kamyczki wrzenne i rozpoczęto proces destylacji. Zebrano do osobnych odbieralników trzy frakcje wrzące kolejno w coraz wyższych temperaturach, a następnie przelano je do parownic i podpalano. Ciecz we wszystkich parownicach uległa zapaleniu, lecz im cięższa frakcja (ma wyższą temperaturę wrzenia) tym trudniej się zapala, a płomień jest coraz bardziej kopcący.
What substances can be extracted from crude oil?
Crude oil is a mixture of numerous chemical substances.
crude oil,
torch,
heating mantle,
250 cmIndeks górny 33 round‑bottom flask,
partial condenser,
boiling chips,
thermometer,
condenser,
3 plugs,
3 conical flasks,
3 porcelain evaporating dishes.
Assemble a distillation set consisting of: a distillation flask, a partial condenser, a thermometer, a condenser and a receiver.
Pour about 100 cmIndeks górny 33 of crude oil to the flask and add several boiling chips to prevent the liquid from overheating.
Heat the flask in the heating mantle.
Collect crude oil distillation products (fractions) into conical flasks at temperature range 40–180°C and then close the flasks with a plug.
Determine the colour and odour of the individual fractions.
Then transfer 1 cmIndeks górny 33 of each distillate to three evaporating dishes and them for flammability using a lit torch.
The importance of products obtained from crude oil
Crude oil is undoubtedly one of the most important raw materials used by humanity due to the high importance of individual components that can be obtained from it. That is why oil refineries work in the so‑called continuous manner. Refinery gases, i.e. a mixture of four first alkanes, are collected at the top of the distillation column. In addition, four fractions are obtained: petrol, kerosene, diesel oil, and mazut.
Fractions with a boiling point above 350°C are distilled under decreased pressure. For example oils, petroleum jelly, paraffin, and asphalt are obtained as a result.
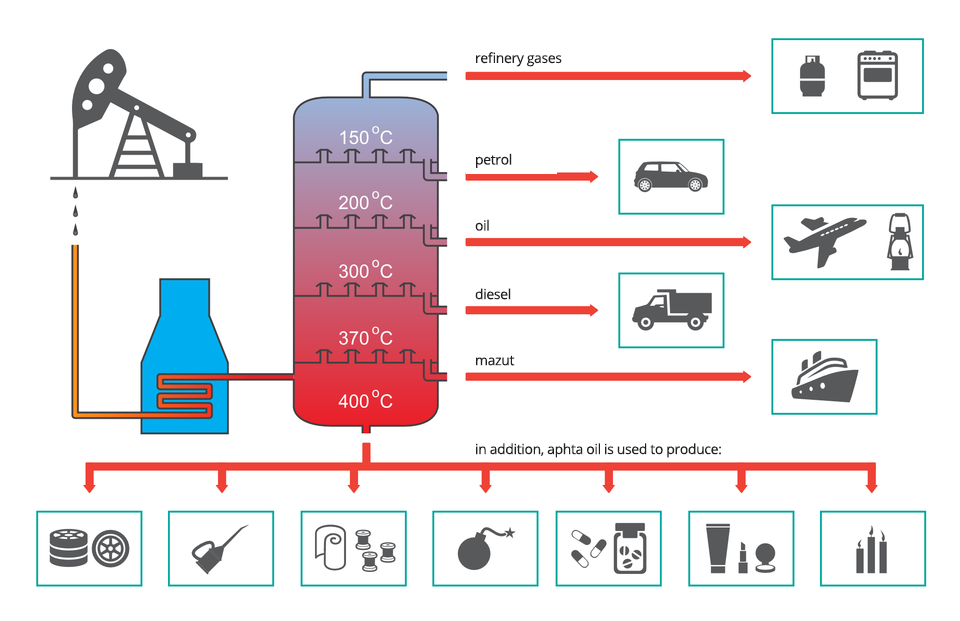
Nowadays no one doubts that oil plays a huge role in the modern world. It is so valuable that fights for access to areas where it occurs often lead to armed conflicts. Products obtained during processing of this raw material surround us every day, starting from vehicles which drive on asphalt roads or fly in the sky and ending with oils, greases, tar, plastics, medicines, rubber, cosmetics, toys, explosives, clothing and other everyday products.
Natural gas
Natural gas is the so‑called blue fuel which is found alone or together with seams of crude oil. It is a mixture of light hydrocarbons the main component of which is methane. It contains also other hydrocarbons as well as nitrogen, carbon dioxide, and very often hydrogen sulphide and helium. Due to the last two components, after extraction, natural gas needs to be purified, because otherwise sulphur dioxide generated in the process would cause dangerous environmental pollution. After desulphurization, it exhibits high energy capacity and is associated with low emission of greenhouse gases and lack of dangerous waste. The importance of this energy resource, considered as the purest source of energy, is constantly increasing.
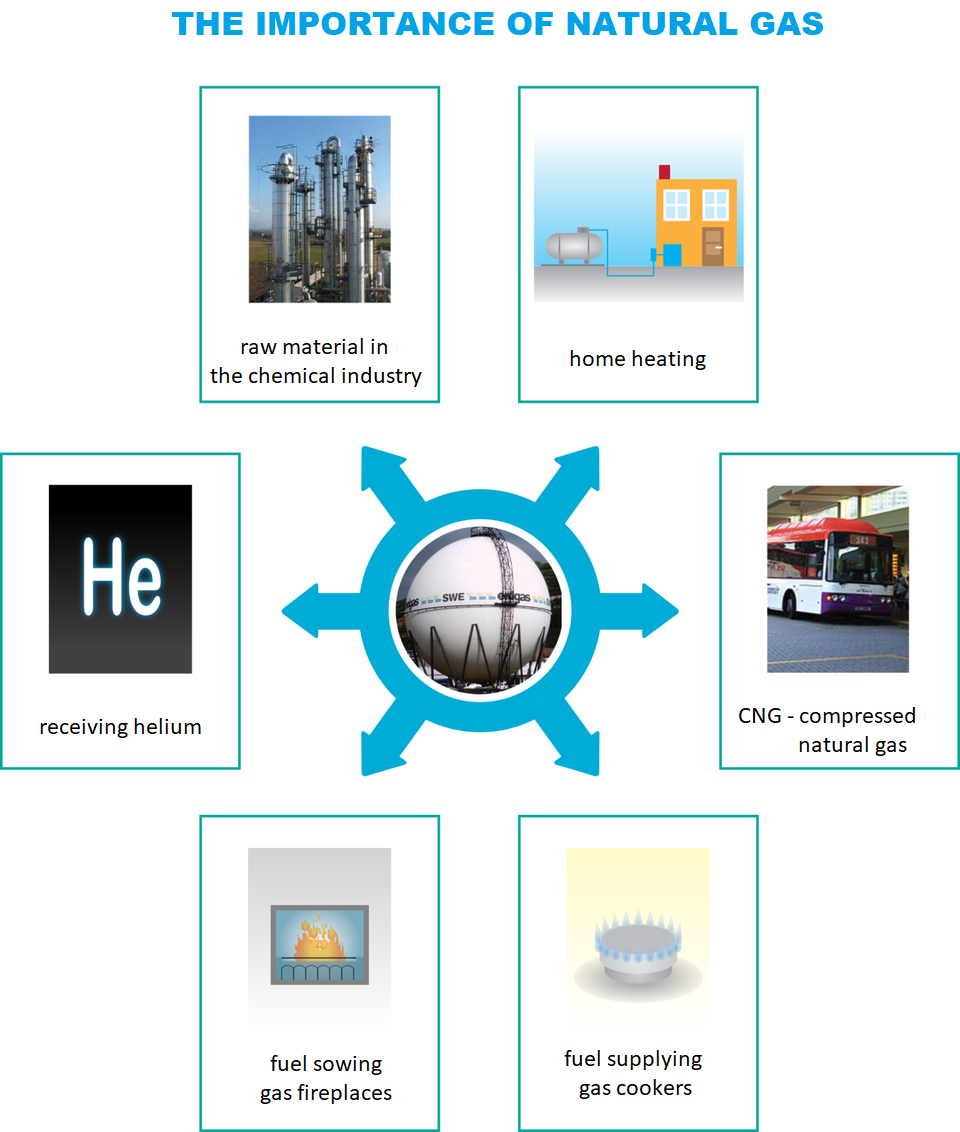
1. the importance of natural gas
2. raw material in the chemical industry
3. home heating
4. obtaining helium
5. CNG - Compressed Natural Gas
6. fuel supplying the gas fireplace
7. fuel supplying gas cookers
Mark true statements.
- A gas called refinery gas is obtained during distillation of crude oil.
- Kerosene is a thick, oily, dark brown or black liquid obtained during distillation of crude oil.
- Mazout is a crude oil fraction containing hydrocarbons made up of carbon chains with 9 to 16 carbon atoms in a particle.
- Crude oil fractions differ from each other with their properties.
- Petrol is obtained during distillation of crude oil at a temperature from 40 to 180°C.
- Ignacy Łukasiewicz invented a kerosene lamp.
- Kerosene is obtained from mazout and it is used to produce candles.
- Large quantities of paraffin are used in aviation as fuel.
Summary
The following natural mineral fuels can be distinguished: hard coal, brown coal, peat, crude oil and natural gas.
The products of fractional distillation of crude oil under normal pressure are as follows: refinery gases, petrol, diesel oil and mazout.
The following products are obtained as a result of distillation of mazout under decreased pressure: oils, petroleum asphalt, tar, petroleum jelly, and paraffin.
Keywords
distillation, fractional distillation, fraction
Glossary
destylacja – metoda rozdzielania składników ciekłej mieszaniny, wykorzystująca różnice w ich temperaturach wrzenia; proces destylacji polega na odparowywaniu kolejnych składników mieszaniny, a następnie skraplaniu ich par w wyniku oziębienia
destylacja frakcyjna (destylacja frakcjonowana, rektyfikacja) – metoda rozdzielania składników mieszaniny wieloskładnikowej na frakcje
frakcja – mieszanina substancji o zbliżonych temperaturach wrzenia, mieszczących się w określonym przedziale wartości
sucha destylacja – proces polegający na termicznym odgazowaniu paliw stałych bez dostępu powietrza