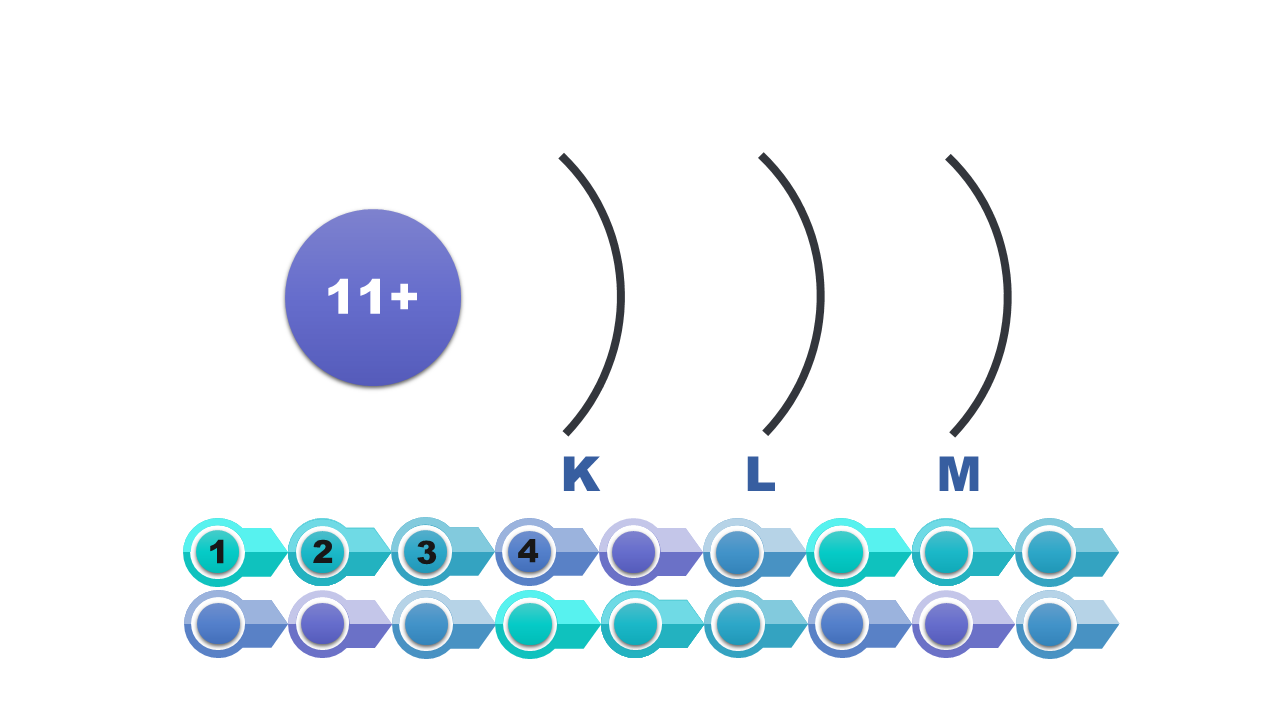
Electrons in atom
that the elements creating the atom are: electrons, protons, neutrons;
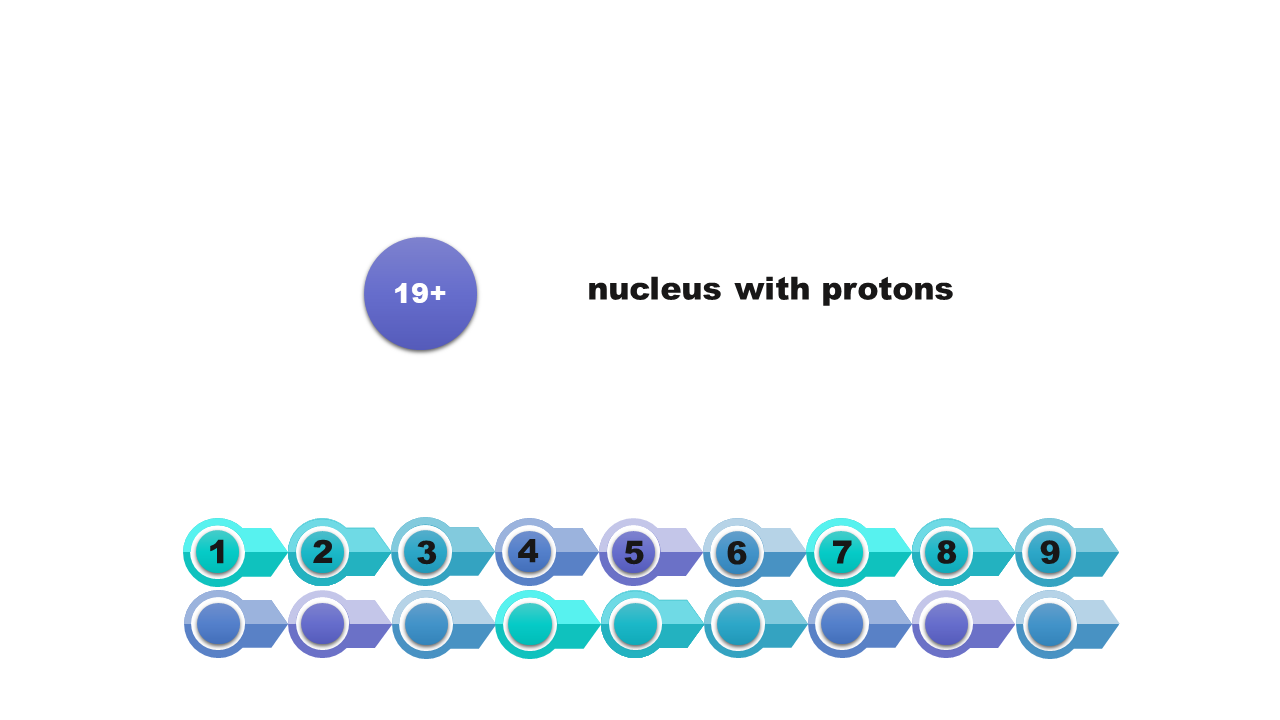
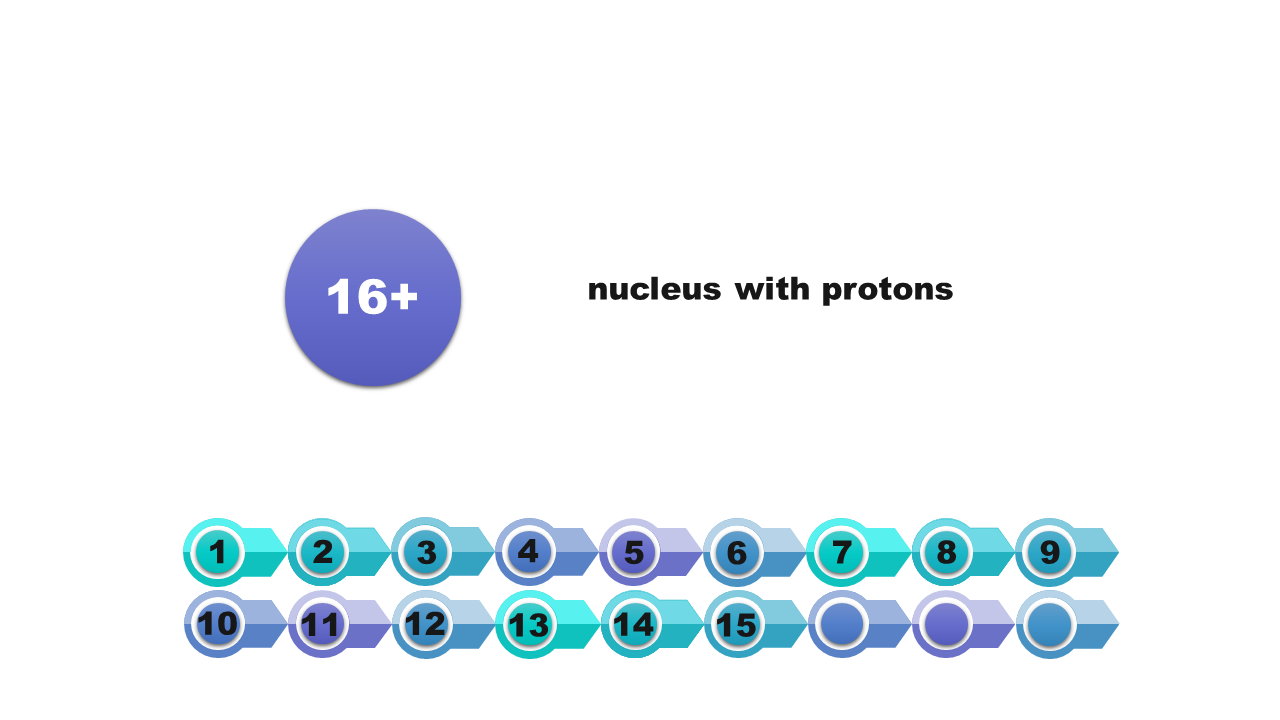
that the central part of the atom (atomic nucleus) is formed by nucleons (protons and neutrons);
that electrons are located in the space around the atomic nucleus;
that the number of protons is equal to the number of electrons in an atom.
mark electron shells in an atom;
determine the maximum number of electrons forming individual atomic electron shells;
describe the configuration of electrons in an atom;
indicate valence electrons.
History of atom
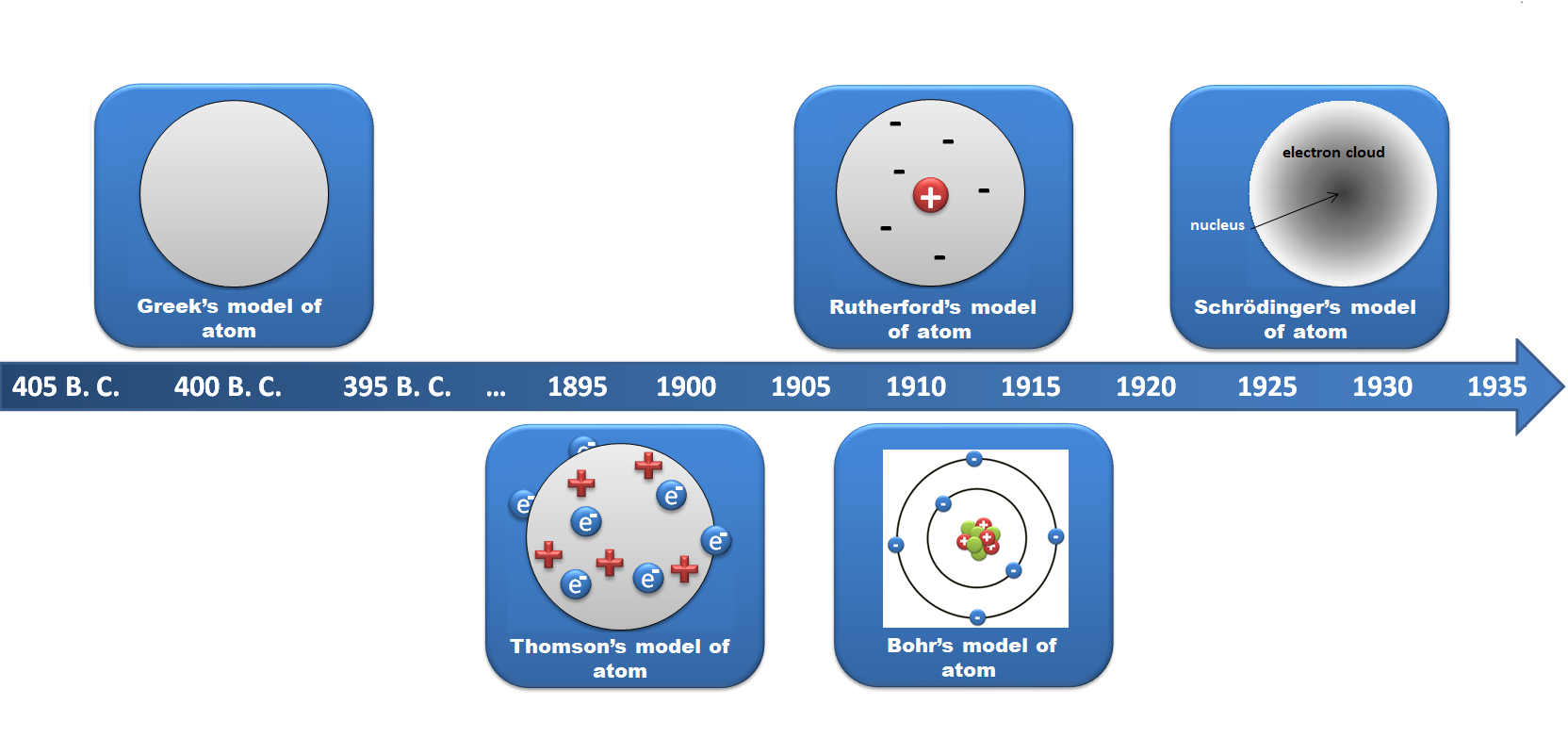
Organize the discoveries regarding the construction of the atomic model
Democritus (400 B.C.) taught that atoms are infinite in number, eternal. Describes atoms as all made of the same substance, and differing only in size, shape and arrangement in space., N. Bohr (1913) model include the electrons move in spherical orbits at fixed distances from the positively charged nucleus., J. Chadwick (1932) Modern model of atom contain positively charged protons and no-charged neutrons in nuclei., J. J. Thomson (1897) proposed that model of atom is like ,,plum pudding’’. He pictured negatively charged electrons embedded in concentric ring in a sphere of positive electric charge., E. Rutherford (1911) proposed that the positive charge of the atom is concentrated in a tiny nucleus at the center of the atom. Electrons move randomly in the space around the nucleus., E. Schrödinger (1926) developed mathematical equations to describe the motion of electrons in atoms. Electrons exist as clouds of electric charge within ,,orbitals’’ that define regions of space with a high probability of containing the electrons.
Bohr proposed that the electrons are arranged in concentric circular orbits around the nucleus. The Bohr model was modeled on the solar system, known as the planetary model.
Generally, the Bohr model can be summed up with the following principles:
Electrons occupy only some orbits around the nucleus. These orbits are stable (stationary orbits).
Each orbit has associated energy. The orbit nearest the nucleus has the energy E1, the next orbits in the order from the nucleus have the energies E2, E3, etc.
Energy is absorbed when the electron jumps from a lower orbit to a higher one and energy is emitted when the electron drops from a higher orbit to a lower orbit.
The energy and frequency of the emitted or absorbed light can be calculated using the difference between the two orbital energies.
The Bohr model concerned mainly the hydrogen atom model and did not explain the structure of atoms more complex than hydrogen. It was not until 1926 that a new, more complete atomic theory was developed - a modern atomic theory.
During the 10 years since the discovery of the Bohr atom model, there has been a great development in this field of science. In 1921 Louis de Brogllie introduced the wave/particle duality of matter. Werner Heisenberg elucidated the Uncertainty Principle in 1923. In 1926 Erwin Schrödinger, an Austrian physicist, took the Bohr atom model one step further. He developed the equation which is used today to understand atoms and molecules - the Schrodinger Equation.
Schrödinger used mathematical equations to describe the probability of finding an electron in a particular position. This atomic model is known as the quantum‑mechanical atom model. In contrast to the Bohr model, the quantum‑mechanical model does not specify the exact path of the electron, but rather predicts the chances of locating the electron. The model is presented as a nucleus surrounded by an electron cloud. Where the cloud is the most dense, the probability of finding an electron is greatest, and vice versa, the electron is less likely in a less dense cloud area. In 1932, James Chadwick discovered that the atomic nucleus consists of positively charged protons and neutron neutral electric charge particles. From 1932, through continuous experiments, many additional particles were discovered in the atom. The atomic theory has been further enhanced by the idea that protons and neutrons even form smaller units called quarks. The quarks themselves are in turn made of vibrating energy strings. The atomic composition theory is a continuous and exciting adventure.
In a quantum (wave) mechanical model, an electron is seen as a standing wave. This is related to a series of wave functions (orbitals) that describe the possible energies and spatial distributions available for the electron. According to Heisenberg's uncertainty principle, the model can not specify detailed electron movements. Instead, it represents the probability distribution of the electron on this orbital. Thanks to this, the image of orbitals is possible due to the probability distribution of electron density maps.
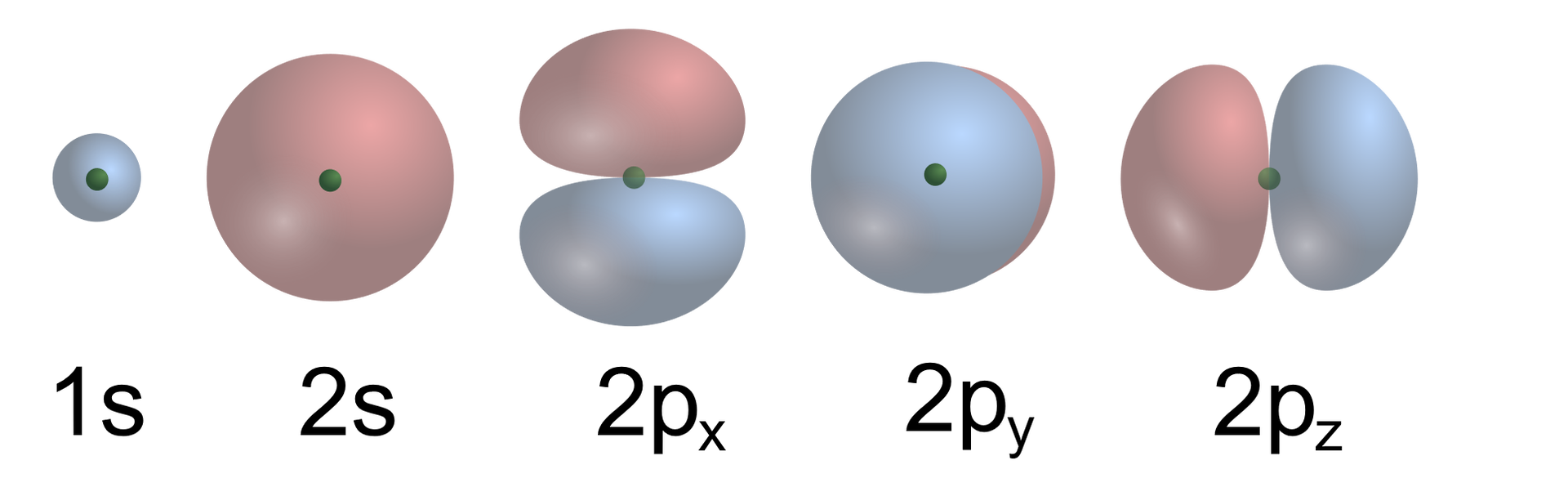
How electrons move?
The electrons occupy the space around the nucleus in the atom. They move there at high speed and in different directions. They are said to create an electron cloud.
The space in the atom occupied by electrons is enormous in relation to the volume occupied by the atomic nucleus. However, this does not mean that each of the electrons moves freely in every point of this space. It turns out that electrons move only in limited areas. These areas are named electron shells. Within them, electrons move at high speed and in all directions. The number of electron shells in atoms varies and depends on the number of electrons. The largest atoms we know have seven shells, and the smallest ones – one.
Electrons moving on different shells differ in energy. The closer the electron is to the atomic nucleus, the lower its energy is. And on the contrary – the farther away from the atomic nucleus electron is, the higher its energy is.
Electron shells are not physically reflected in the structure of the atom. It is primarily the energy of a given electron and the presence of other electrons that determine in which area around the nucleus it will move. There are no physical barriers in the space around the nucleus that would hold the electron on a given shell.
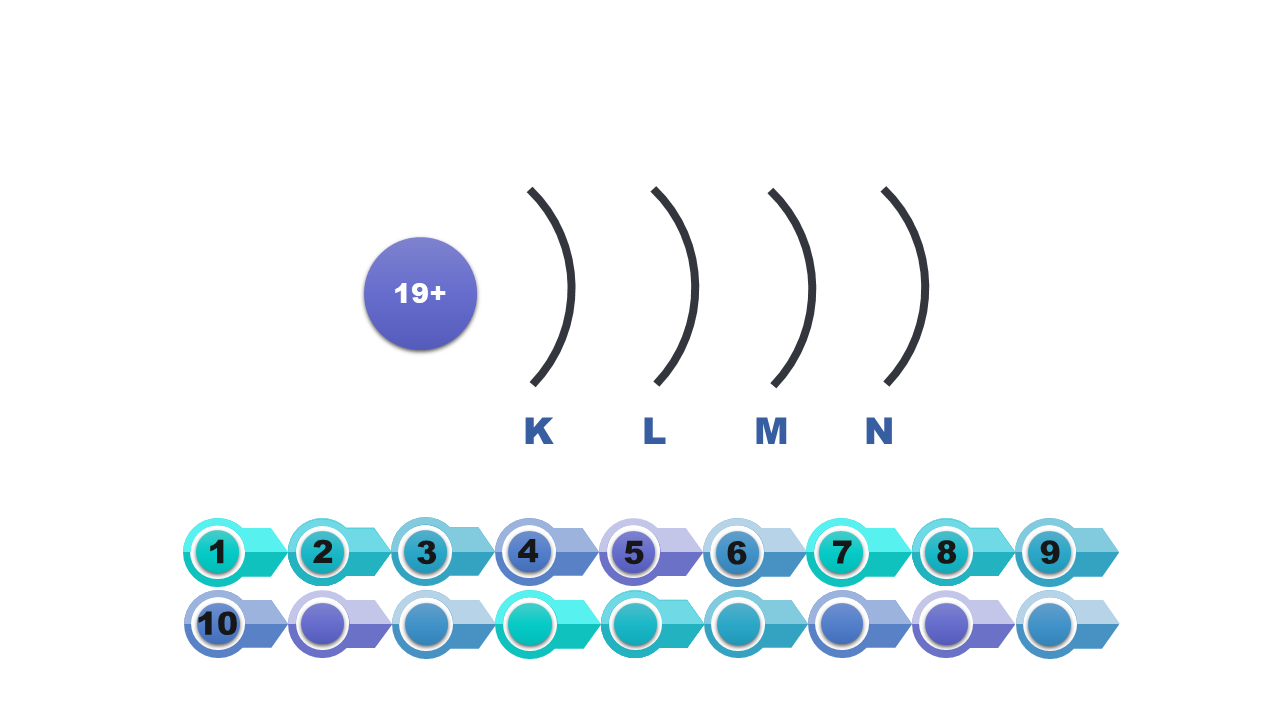
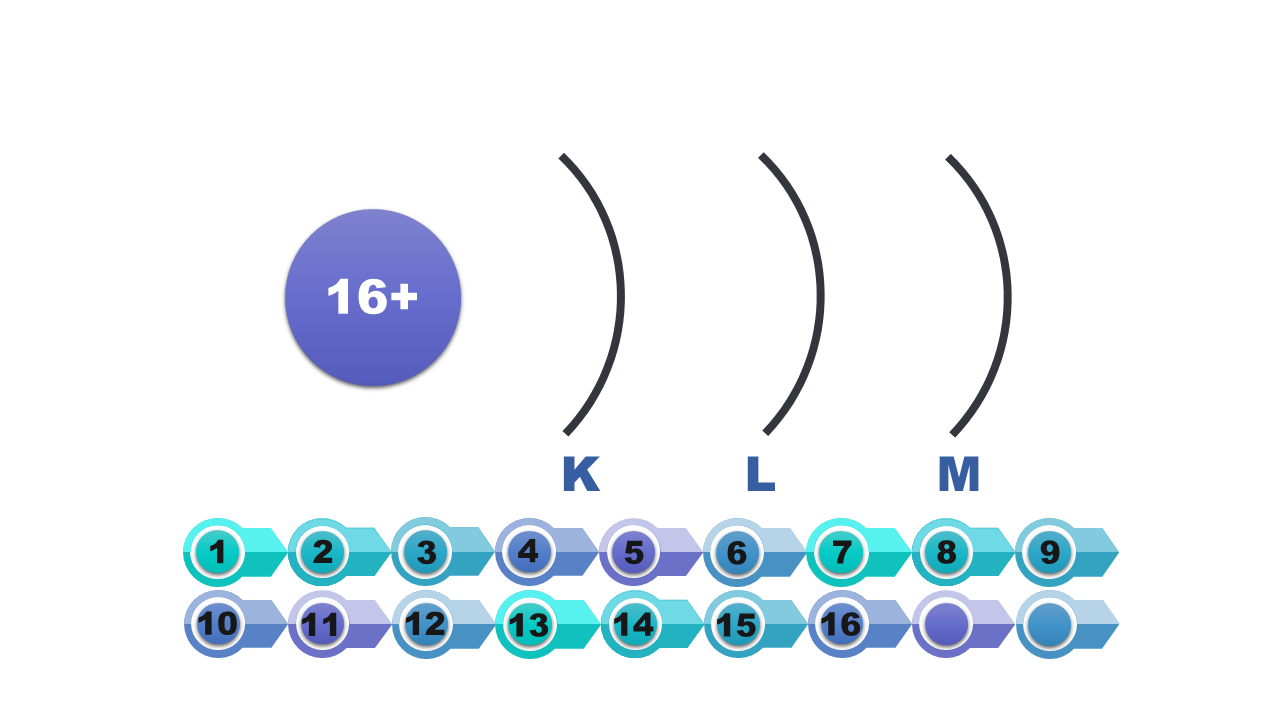
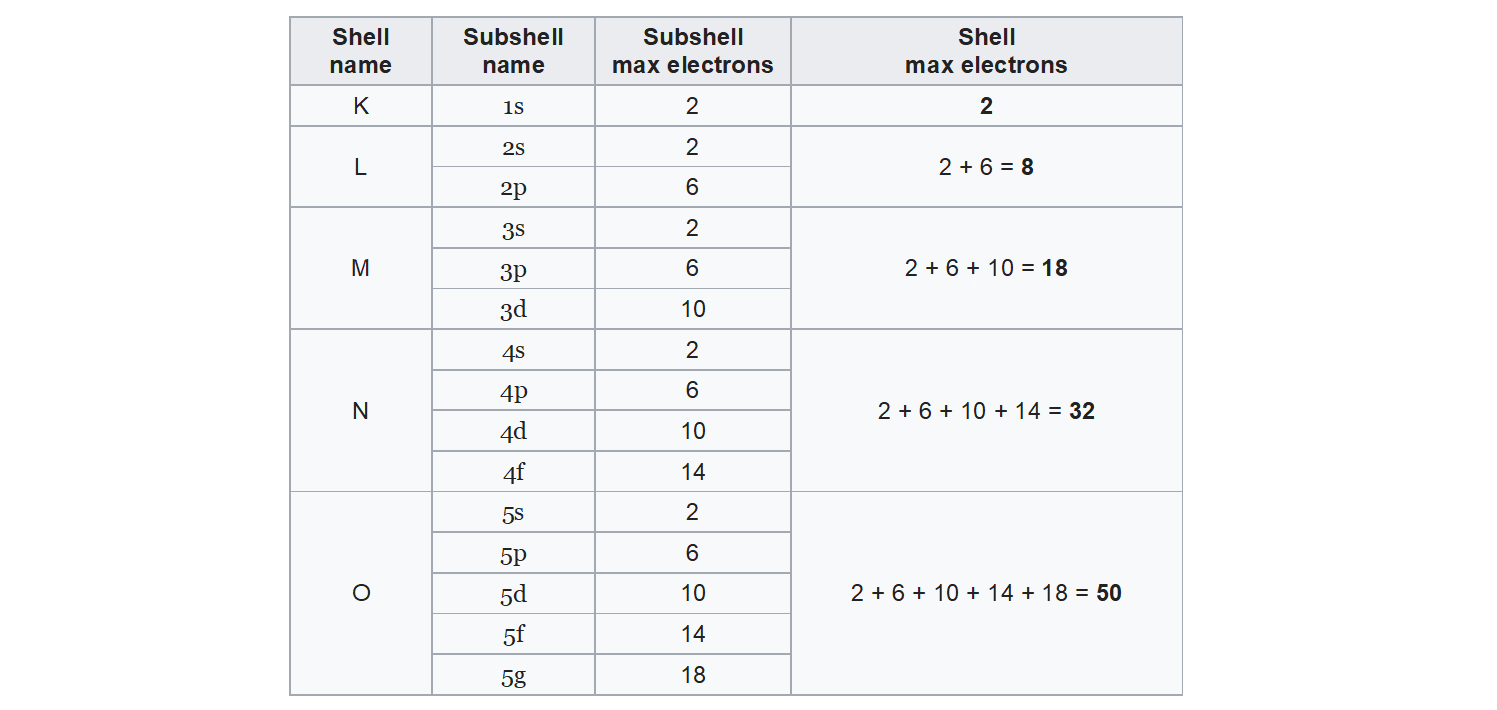
An electron shell around a given atom is considered a set of atomic orbitals having the same major quantum number n. The next n values are assigned to subsequent shells: K, L, M, N, O, P and Q. The shells consist of different numbers of electron subcoatings, corresponding to certain types of atomic orbitals.
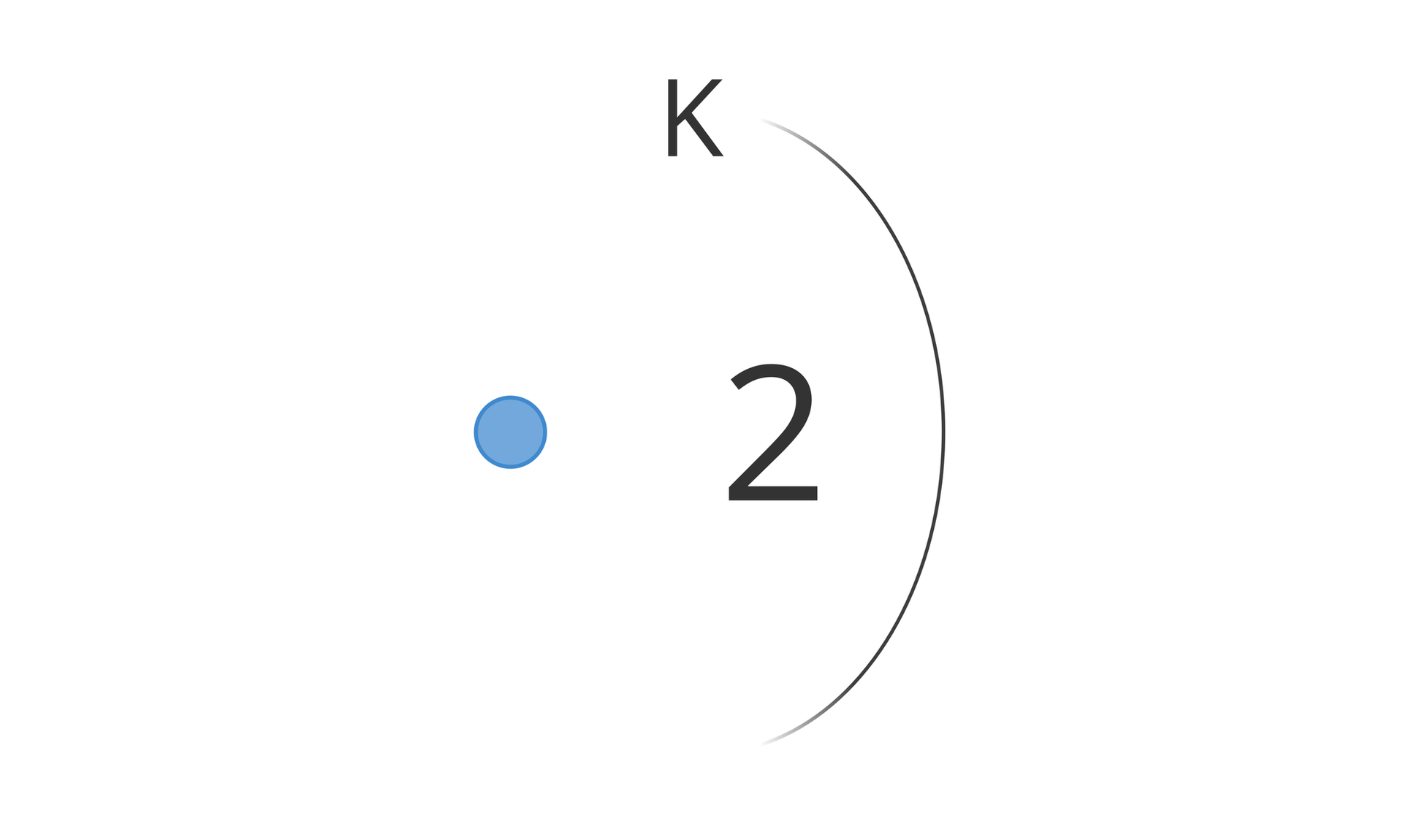
Electrons on shells
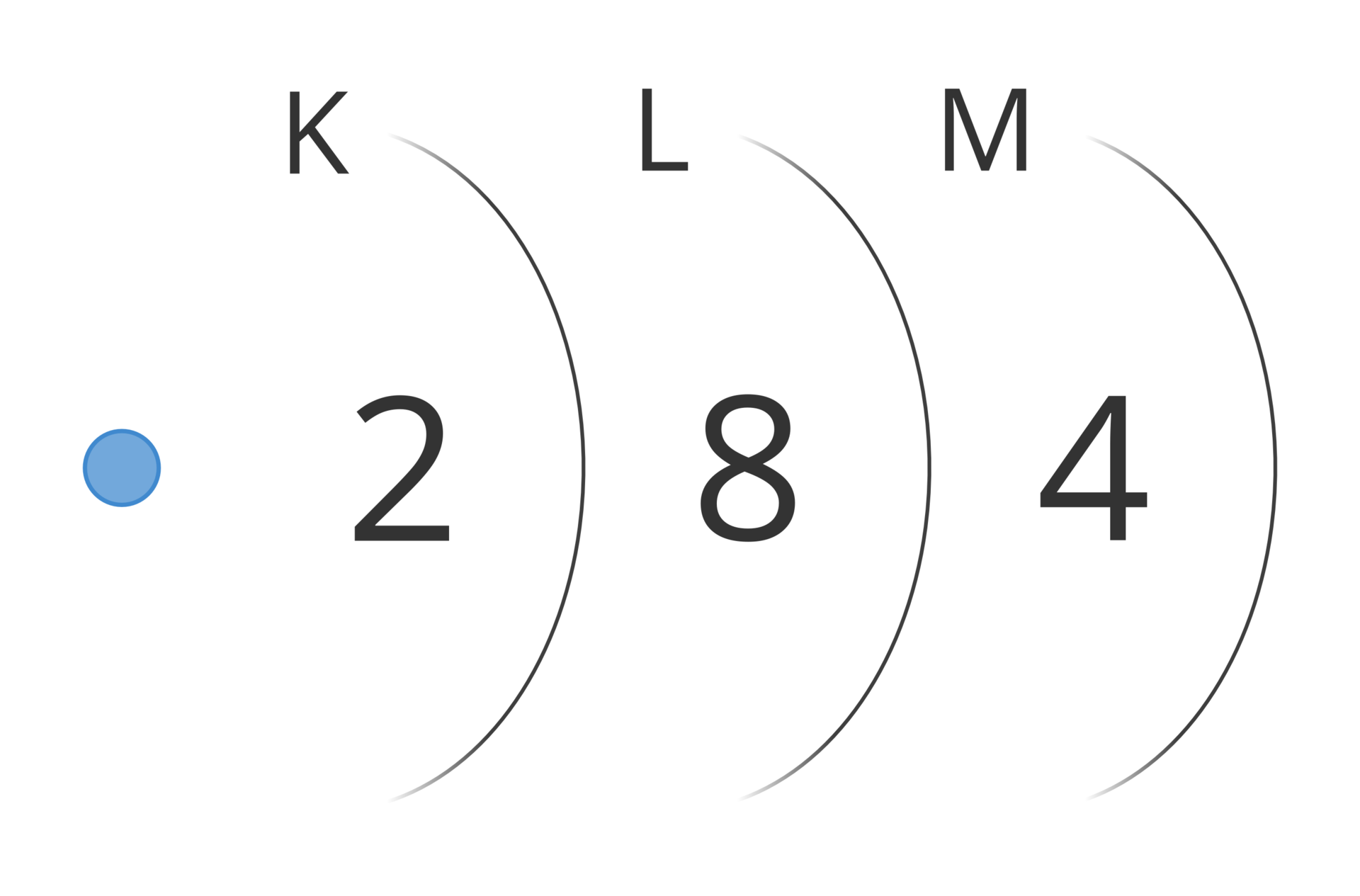
Electron shells in the atom have been given letter symbols from K to Q. The shell closest to the nucleus (the first one) is marked with the letter K. Next are: L, M, N, O, P, Q.
Sequence of shells (distance from the nucleus) | first | second | third | fourth | fifth | sixth | seventh |
Symbol of the shell | K | L | M | N | O | P | Q |
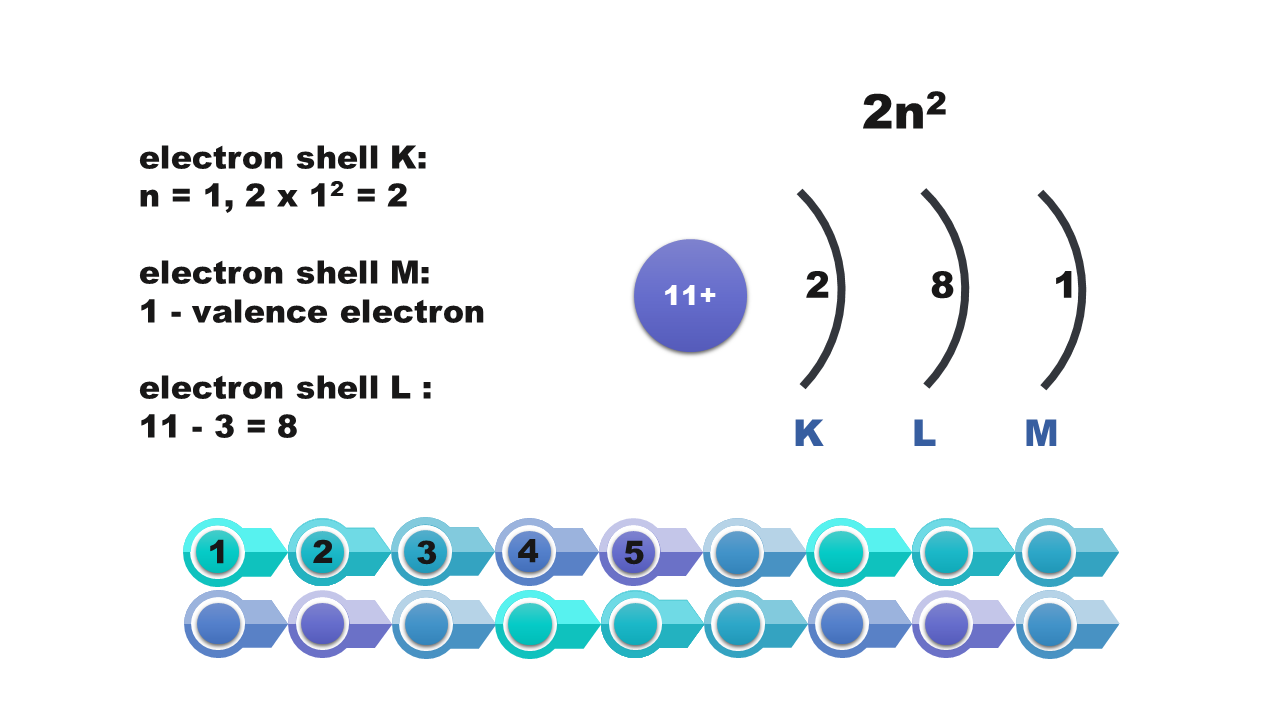
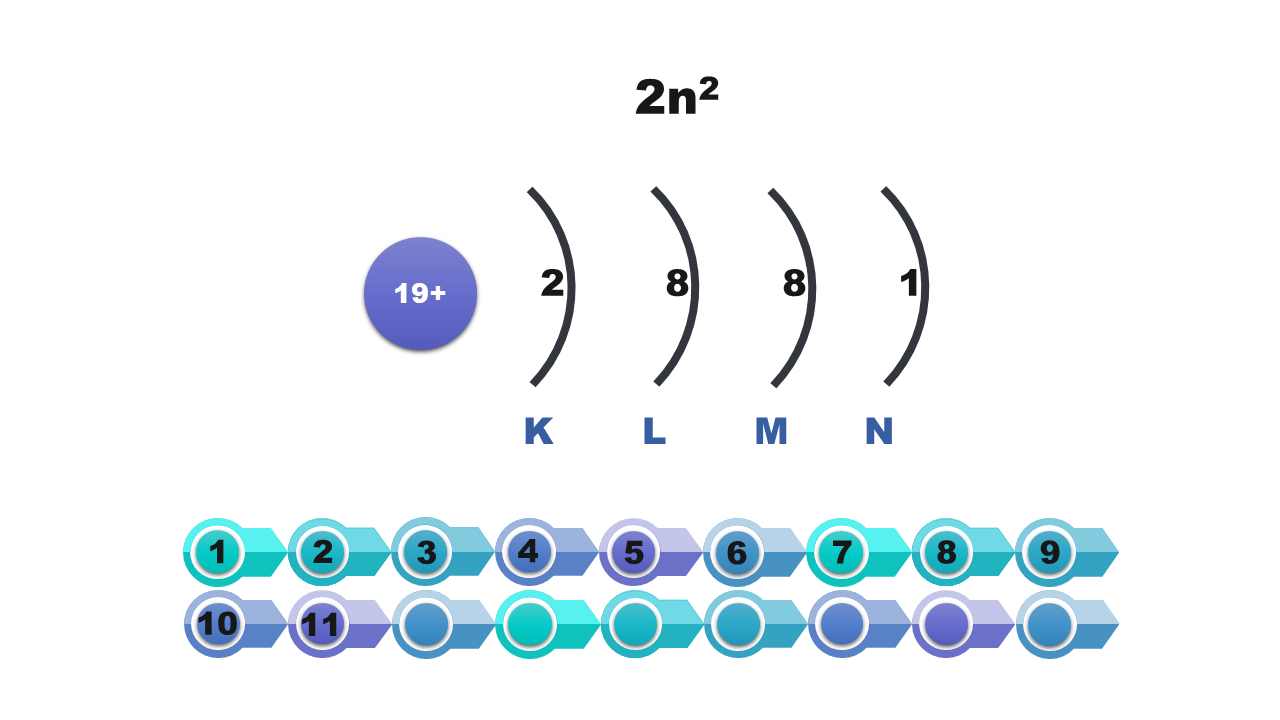
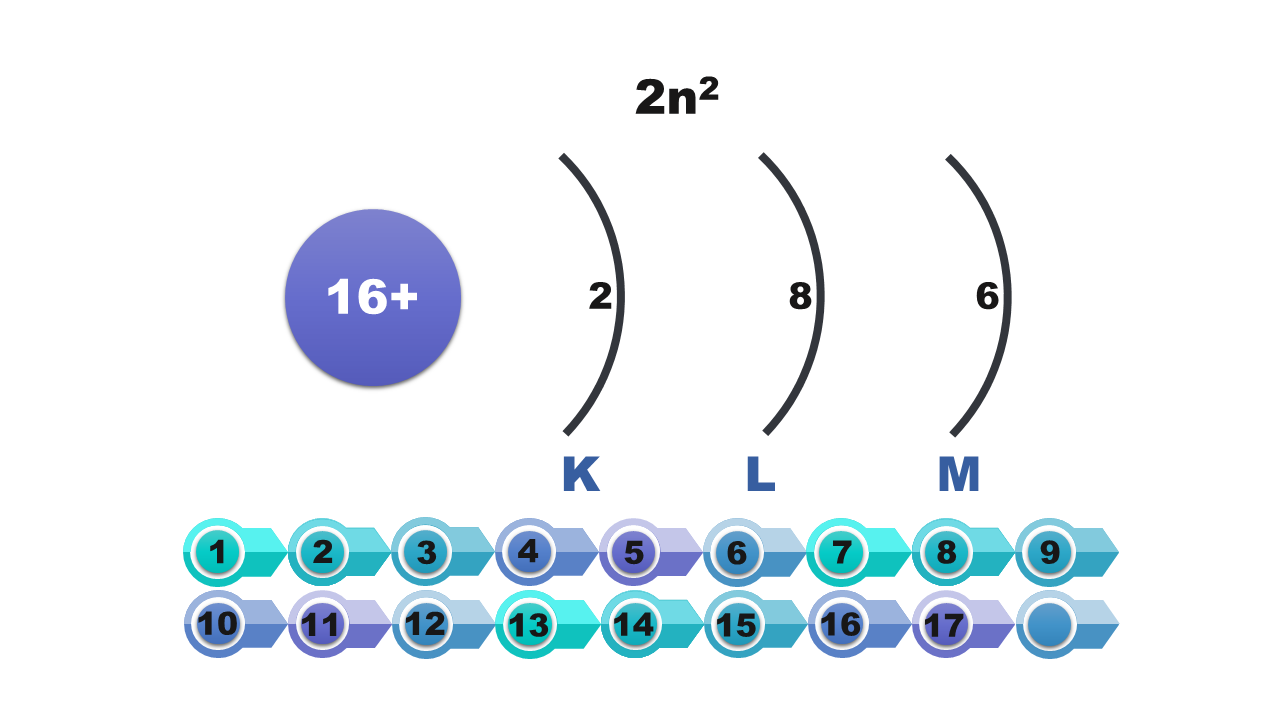
A specified number of electrons can be found on each shell. For example, the first shell holds only two electrons, and the third one can hold only eight of them. The further away from the nucleus of the atom is the shell, the more electrons it can hold. The maximum number of electrons that can be found on the shell is described by the formula 2nIndeks górny 22, where n means the number of the shell.
Number of shell (n) | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
Symbol of the electron shell | K | L | M | N | O | P | Q |
Maximum number of electrons per shell (2nIndeks górny 22) | 2 | 8 | 18 | 32 | 50 | 72 | 98 |
Configuration of electrons
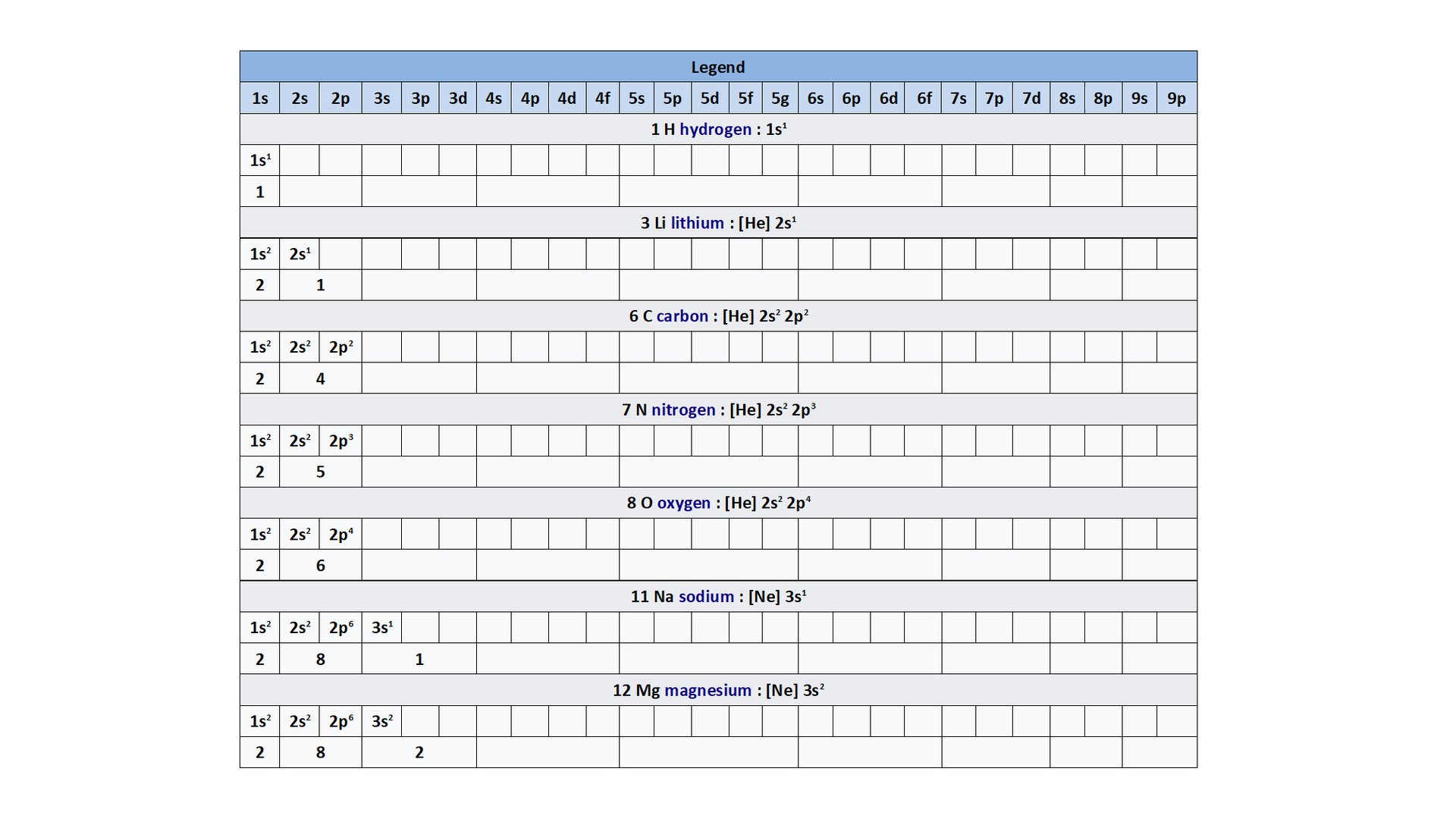
The arrangement of electrons on individual shells is called electron configurationelectron configuration. Presentation of the electron configuration of the atom will start with a helium atom that has two electrons. These two electrons can be located on the first K shell. This information can be presented in several ways. These are presented in the table below.
Type of presentation | Electron configuration | General rules of the notation |
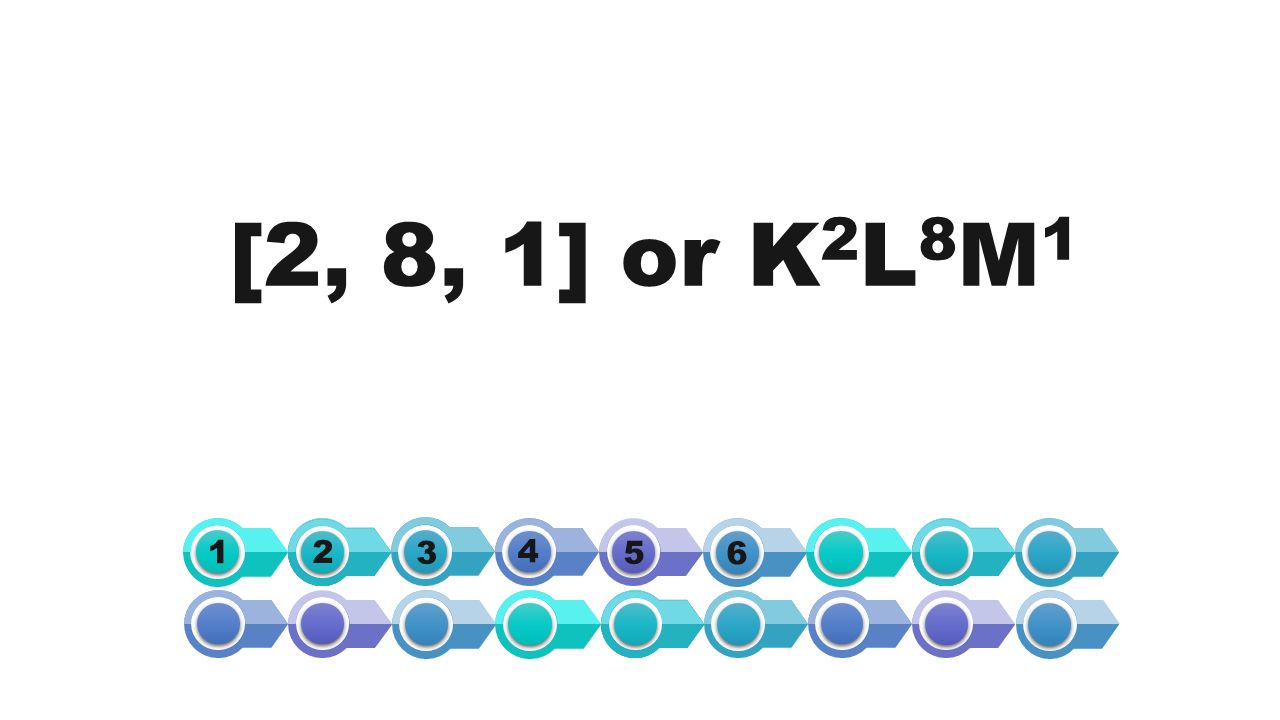
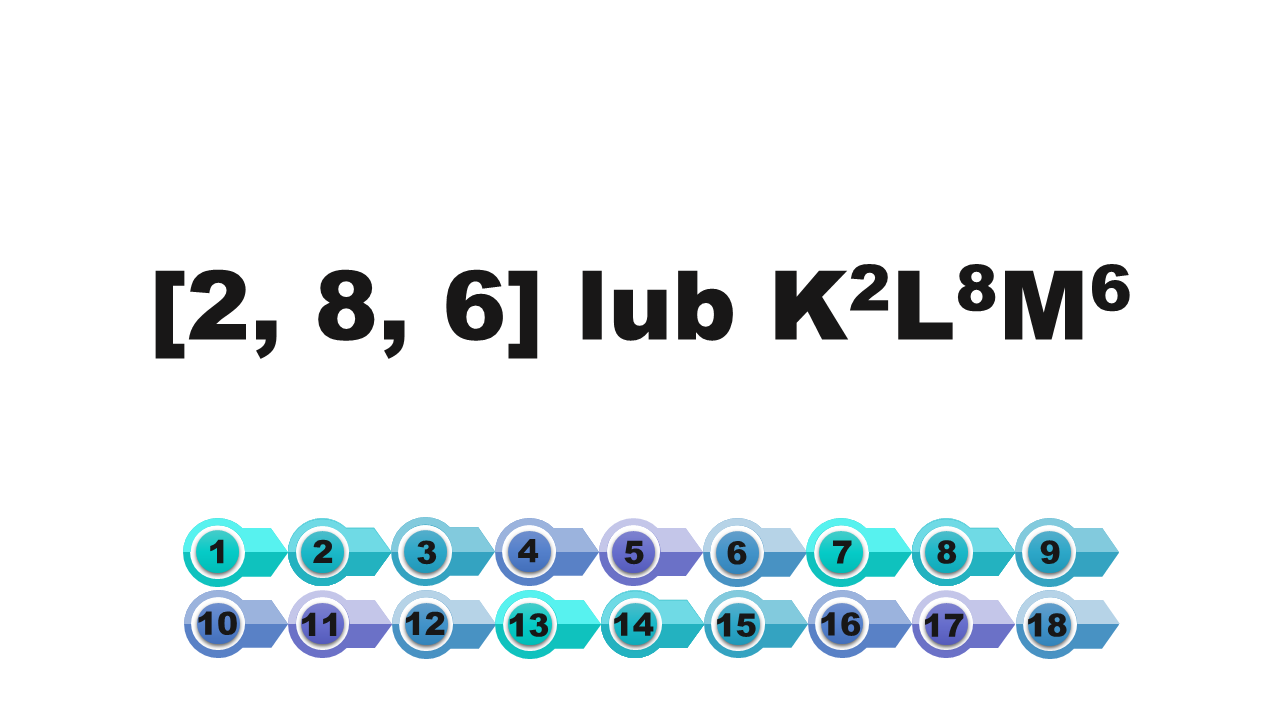
writing using square brackets | [2] | In square brackets we write the numbers of electrons on the first, second and subsequent shells. These numbers are separated by commas. |
writing using shell symbols | KIndeks górny 22 | We note the symbols of the shells occupied by electrons. On the right side of each symbol, in the upper index, we write the number of electrons on the shell. |
Writing in the form of the scheme
We draw a diagram on which we mark the nucleus of the atom and all shell filled with electrons. We note down the symbols of the shells and the number of electrons assigned to them.
Electron configurations of elements on shells and orbitals
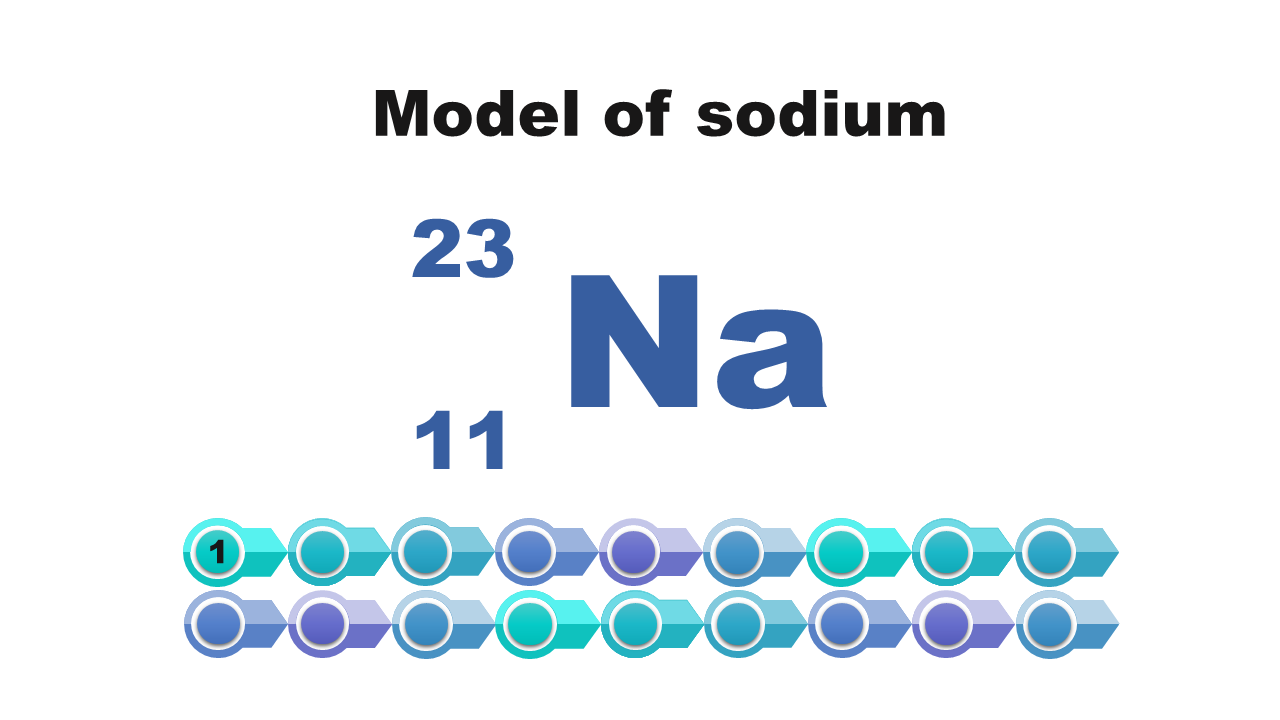
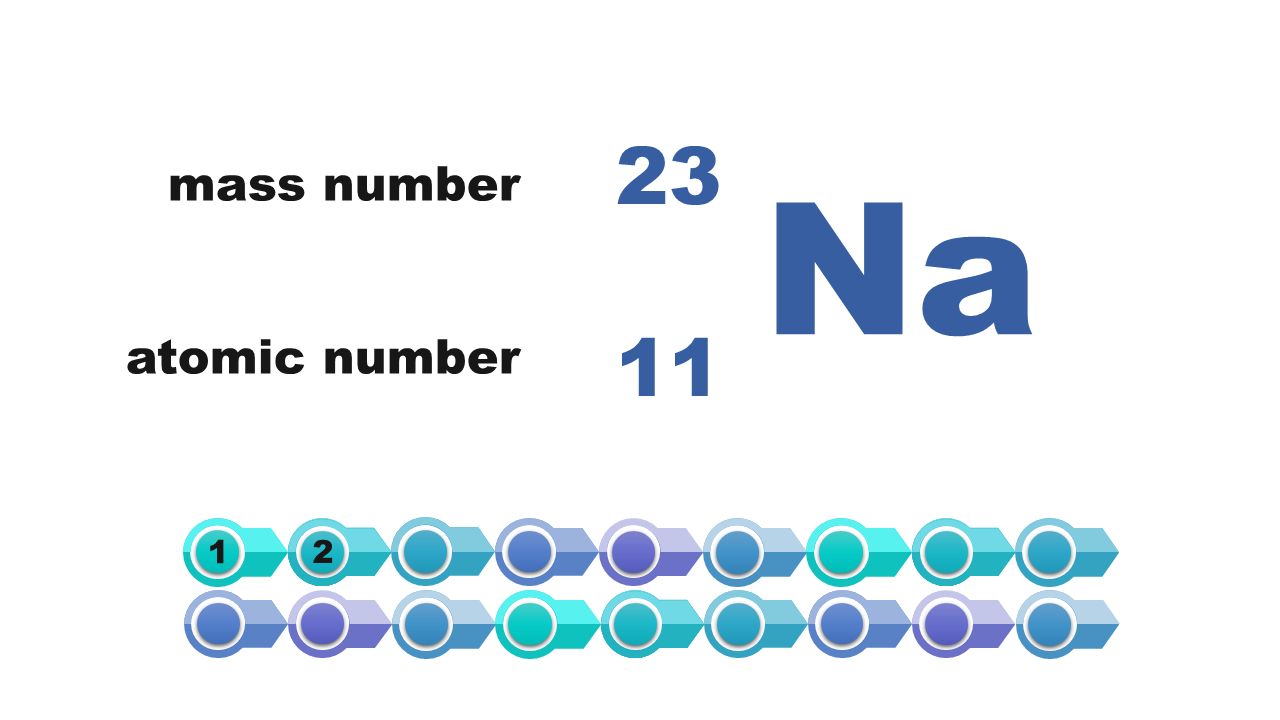
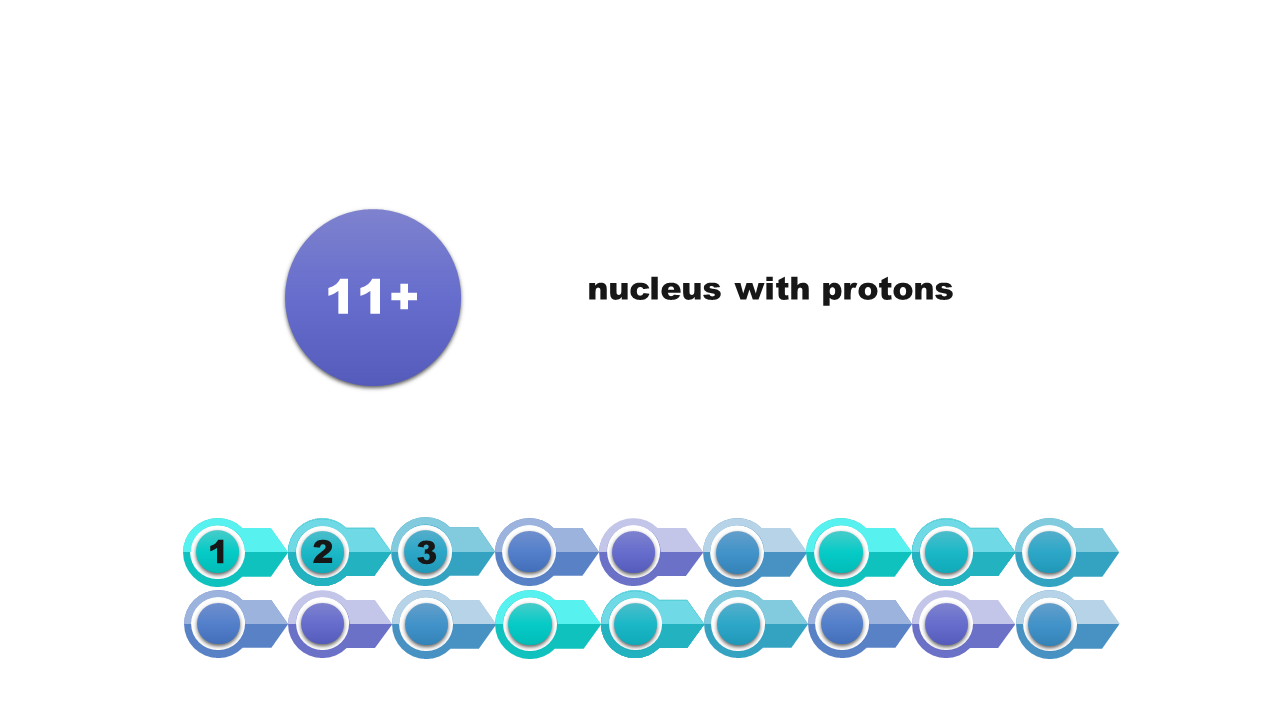
Watch the presentation „Stages of drawing a simplified model of the atom of a chemical element” and pay attention to how the configuration of electrons on electron shells is determined. Remember what further actions should be taken to draw the model of the atom of element. When do we note the electron configuration, creating such a simplified model? Write down the answer.
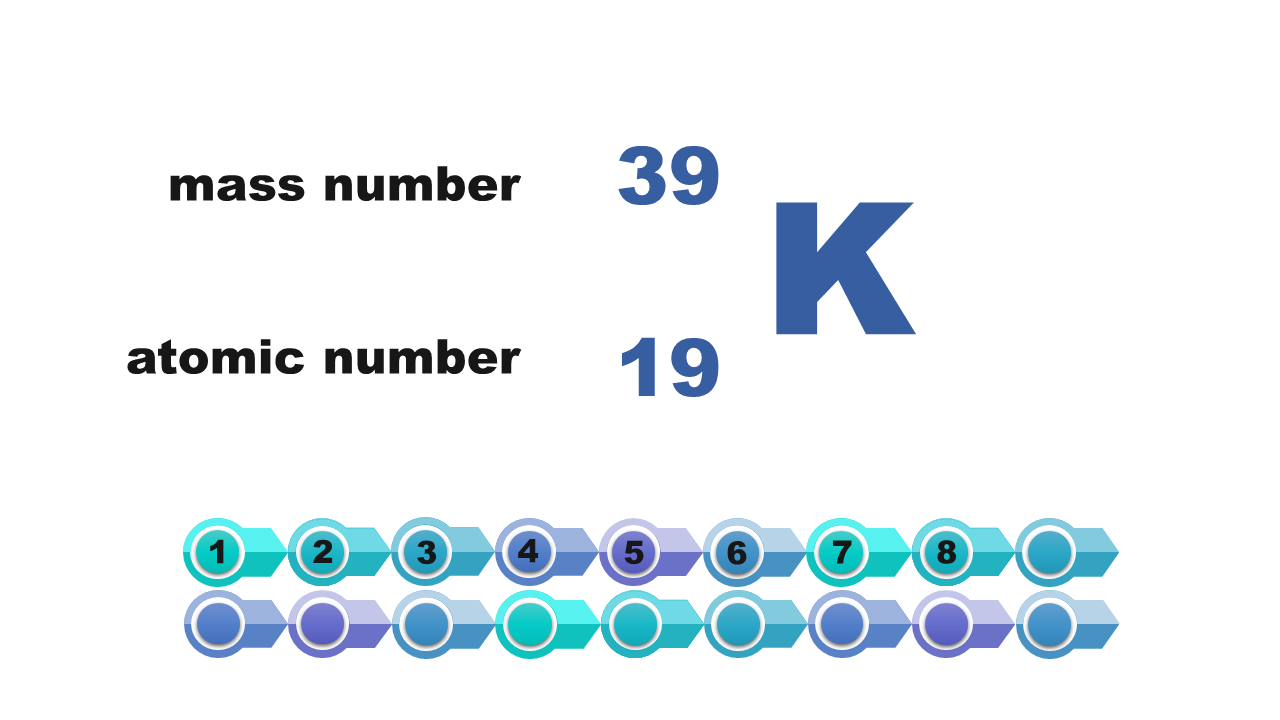
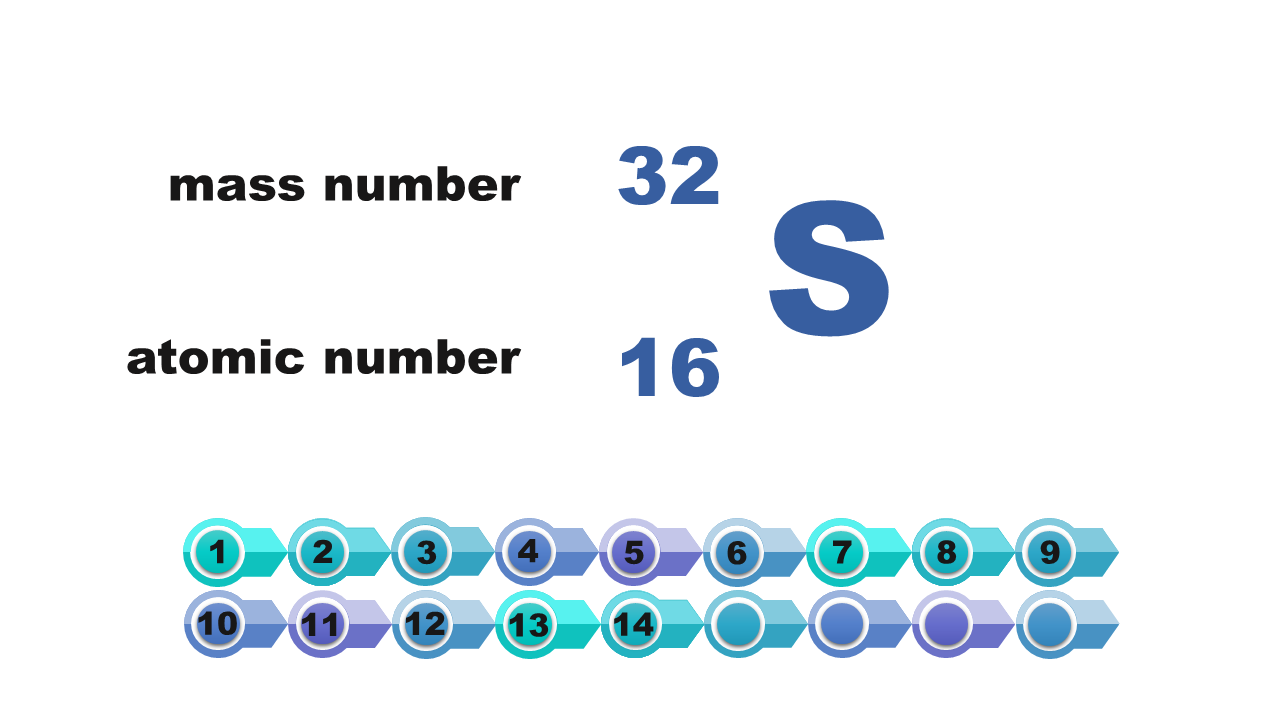
Consider it and then answer which of the numbers – atomic or mass – is necessary to determine the electron configuration of the atom? Write down the answer.
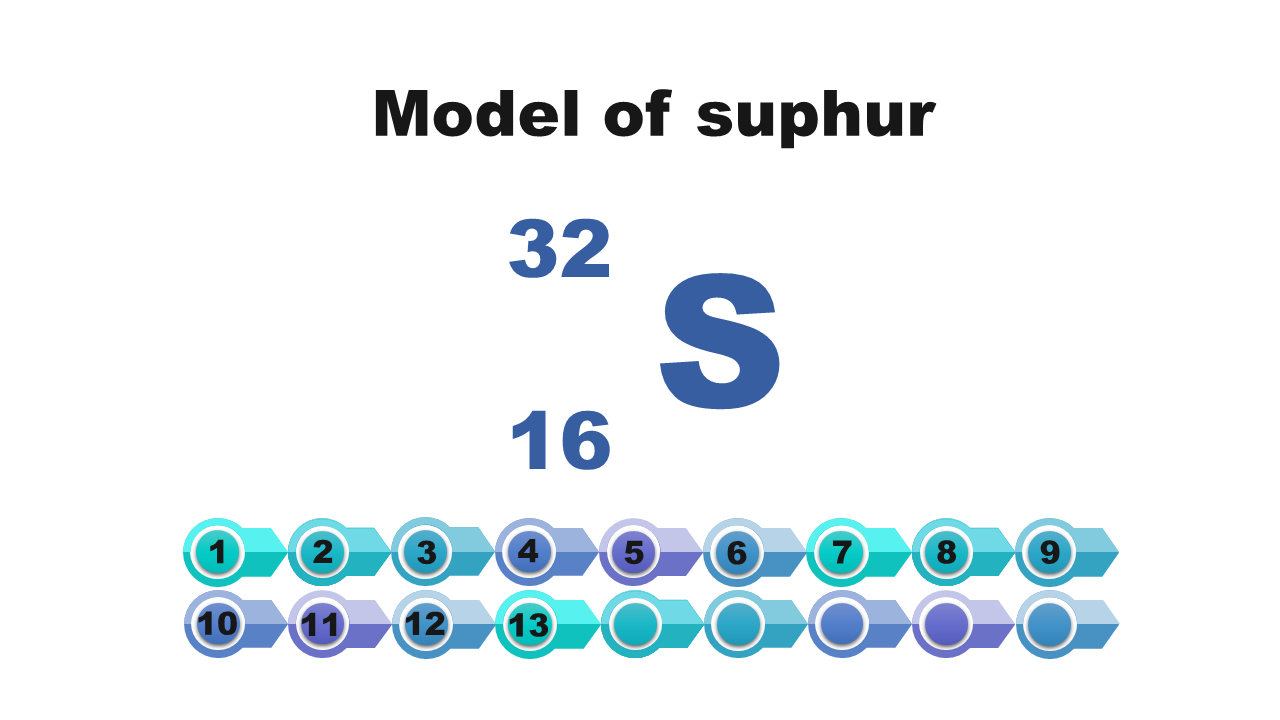
Electron configuration of the silicon atom
Write down using square brackets [2,8,4]
Write down using shell symbols K Indeks górny 22L Indeks górny 88M Indeks górny 44
Writing in the form of the scheme
Filling the 2. (L) and 3. (M) shell in atoms occurs when the lower shell is filled with the maximum number of electrons. In the case of atoms with an atomic number greater than 18, this rule usually does not apply. Although there may be a maximum of 18 electrons on the third shell, the filling of the fourth shell often occurs before the third shell is completely filled.
This phenomenon is illustrated by properly noted electron configurations, including the following atoms of chemical elements:
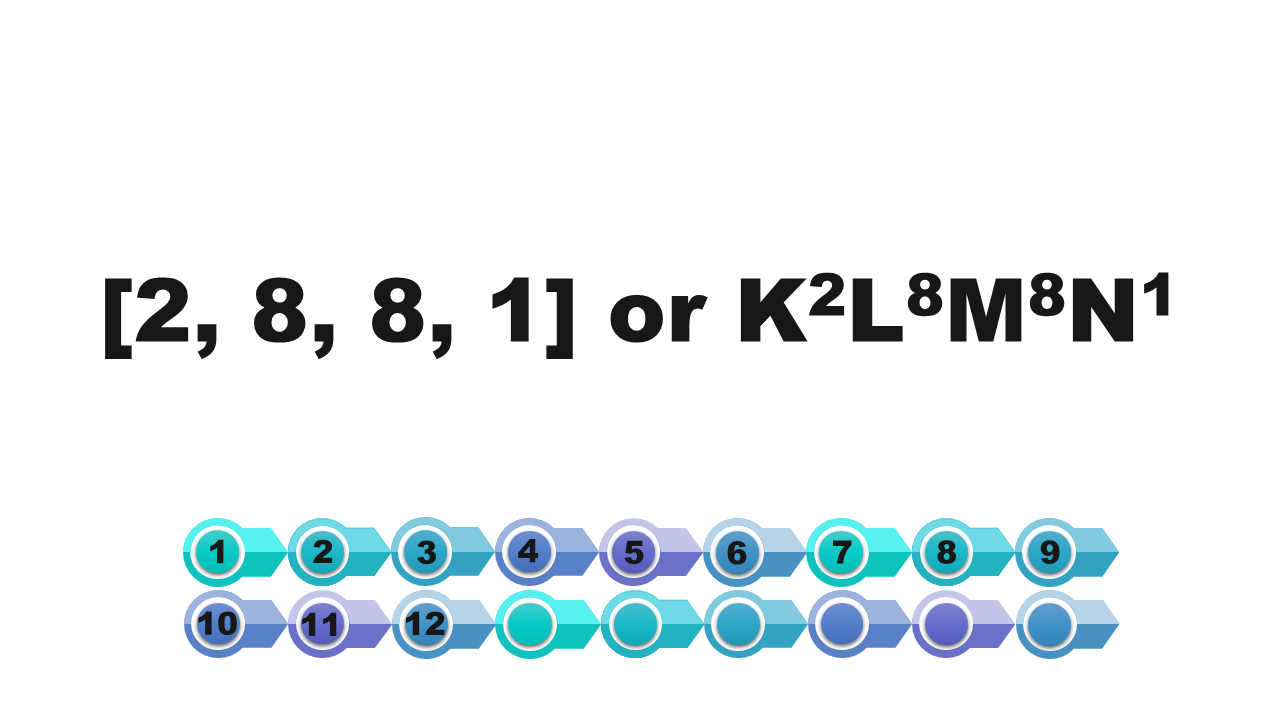
[2, 8, 8, 1]
[2, 8, 8, 2]
[2, 8, 9, 2]
Valence electrons
The electrons farthest from the atomic nucleus are the least attracted by the nucleus and often affect the electrons of other atoms. It can be said that these represent an atom outside. These determine the properties of the atom. As the only one of all of the electrons, these have their own name – valence electronsvalence electrons, and the shell on which these are located is called valence shellvalence shell. Atoms can have a different number of valence electrons (from one to eight).
Atoms | Electron | Number of valence |
KIndeks górny 11 | 1 | |
KIndeks górny 22 LIndeks górny 55 | 5 | |
KIndeks górny 22 LIndeks górny 88 MIndeks górny 44 | 4 | |
KIndeks górny 22 LIndeks górny 88 MIndeks górny 88 | 8 |
Atom of calcium has .... on the M shell:
- 2 electrons
- 8 electrons
- 18 electrons
- 32 electrons
Atom of boron has .... on the last shell:
- 3 electrons
- 2 electrons
- 13 electrons
- 8 electrons
Atom of copper is constructed of:
- 1 electron shell
- 3 electron shells
- 4 electron shells
- 5 electron shells
Summary
The electrons in the atom circulate in a strictly defined space around the nucleus (in areas known as electron shells).
Each shell can accommodate a limited number of electrons (2nIndeks górny 22, n – shell number).
The arrangement of electrons in an atom is called electron configuration.
The last shell in the atom is called the valence shell, and the electrons moving in its space are valence electrons.
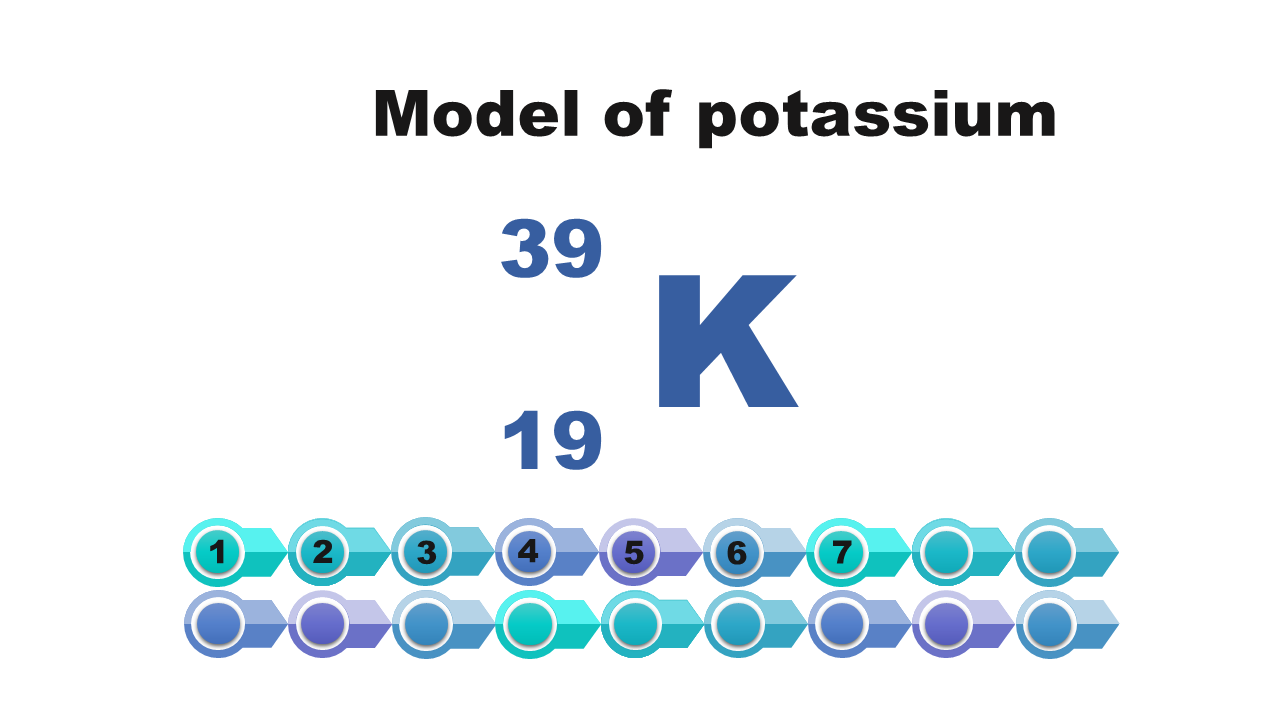
Which of the atoms described has the highest number of valence electrons? Enter this number.
Atom number | 1 | 2 | 3 | 4 |
Atom description | nucleus of atom of sodium consists of 11 protons | mass number of atom = 19 |
Key words
Valence electrons, electron configuration, valence shell
Glossary
elektrony walencyjne – electrons moving in the outer (often farthest from the atomic nucleus, the last) electron shell in the atom
konfiguracja elektronowa – location of the electrons in the atom
powłoka walencyjna – the shell where the valence electrons are located, often the last (most external) electron shell in the atom