Glass - the material of the future with traditions
that carbonates undergo decomposition after prolonged heating;
that some basic oxides react with acid oxides to form salts (the only product of this reaction).
investigate glass properties;
name types of glass;
describe glass manufacture process;
justify the application of different types of glass.
What is glass?
Glass is an amorphous material whose main component is silicon dioxide. These are obtained as a result of melting quartz sand, calcium carbonate and sodium carbonate at a temperature of about 1500°C. The glassy mass cools down without crystallization. The distribution of the basic structural elements of the glass differs from the structure of the quartz and resembles the distribution of particles in the liquid. Unlike liquid, these particles are not able to move freely due to the high viscosity of this material. The glass has an amorphous structure.
Properties of glass
Window plate glass, glasses, plates and other utensils, car windows or various decorations – these are just a few examples of everyday glass. Special glass is used for the production of large telescope mirrors and microscopes or, for example, shower cubicles. In the walls of buildings, architectural glass and glass bricks are installed, and in fireplaces – heat‑resistant glass. Bullet‑proof glass is resistant to impacts of bullets, e.g. from machine guns. The properties of glass are determined by various factors that can be changed to create new products. These include: glass composition, its structure, surface and types of thin layers applied to this surface. In everyday life, we mostly use colourless glass. Coloured glass can be obtained by adding heavy metals, i.e. iron, chromium, manganese, nickel, copper and others to the glass mass (during its melting).
Before you watch the movie „Testing electrical conductivity of glass” and conduct an experiment, formulate a research question and hypothesis. Consider which of the glass features have been shown and think about how this can relate to the application of this material in many areas.

Film dostępny na portalu epodreczniki.pl
Film przedstawia eksperyment: testing the current conductivity through glass (czyli testowanie przewodności prądu przez szkło). Do przeprowadzenie eksperymentu potrzebne są: glass, burner, electric cables, light bulb and flat battery (czyli szkło, palnik, kable, żarówka i płaska bateria). Przebieg eksperymentu: za pomocą palnika podgrzej szkło podpięte do kabli połączonych z baterią i żarówką. W wyniku nagrzewania żarówka powinna się zapalić.
What are the physical properties of glass?
The glass is characterized by low resistance to various mechanical factors and temperature changes. It does not conduct electricity and heat.
burettes,
brick,
piece of thick fabric,
hammer,
burner,
beaker with cold water,
flat battery,
bulb in lamp‑holder adapter,
wires with crocodile clips.
Wrap the burette tightly with a cloth, and then check the strength of burette under compression, stretching and bending. Put it on a brick and hit it with a hammer. Look at the glass pieces obtained, compare their size and pay attention to the edges.
Put one end of the burette into the flame of the burner and immerse it in a beaker filled with cold water.
Put the burette into the flame of the burner and after a while, check if it can be bent and stretched. Pay attention to the change in the colour of the flame during heating.
Build an electrical circuit, consisting of: a flat battery, a bulb in lamp‑holder adapter, electrical wires with crocodile clips. Place a burette in it. Observe the electric current flows after the circuit is closed, i.e. whether the bulb has turned on or not.
A horseshoe‑shaped glass viewing platform protruding 20 m beyond the edge of a cliff, was created in the Grand Canyon of Colorado. Think about it and answer following questions:
What requirements must be met by the glass to build such an object?
Why on the platform can be a maximum of 20 people? Recall the experiment from the previous lesson, during which we compared the hardness of window glass and quartz. What hardness in the 10‑degree Mohs hardness scale has glass (window), and what – quartz?
Why should you put on special shoe protectors before entering this platform?

The compressive strength of the glass is very high. It is 1000 N/ mmIndeks górny 22, or 1000 MPa, which means that breaking a glass cube with an edge length of 1 cm requires a load of 10 tons.
Before you conduct the experiment “Research on glass reactivity”, formulate a research question and hypothesis. Note down how glass behaves against water, acids and hydroxides.
Testing glass resistance to water, acids and hydroxides.
Glass is a material resistant to water, acids and hydroxides.
pieces of broken glass (from previous experiment),
4 transparent polypropylene containers,
hydrochloric acid,
concentrated sodium hydroxide solution,
hydrofluoric acid solution,
water.
Put pieces of glass into each of the four containers.
Add water to the first container, to the second container – concentrated sodium hydroxide solution, to the third – approx. 10% hydrochloric acid, and to the fourth – hydrofluoric acid solution.
Put on the lid and close the containers and leave the test samples for a few days.
Glass types and applications
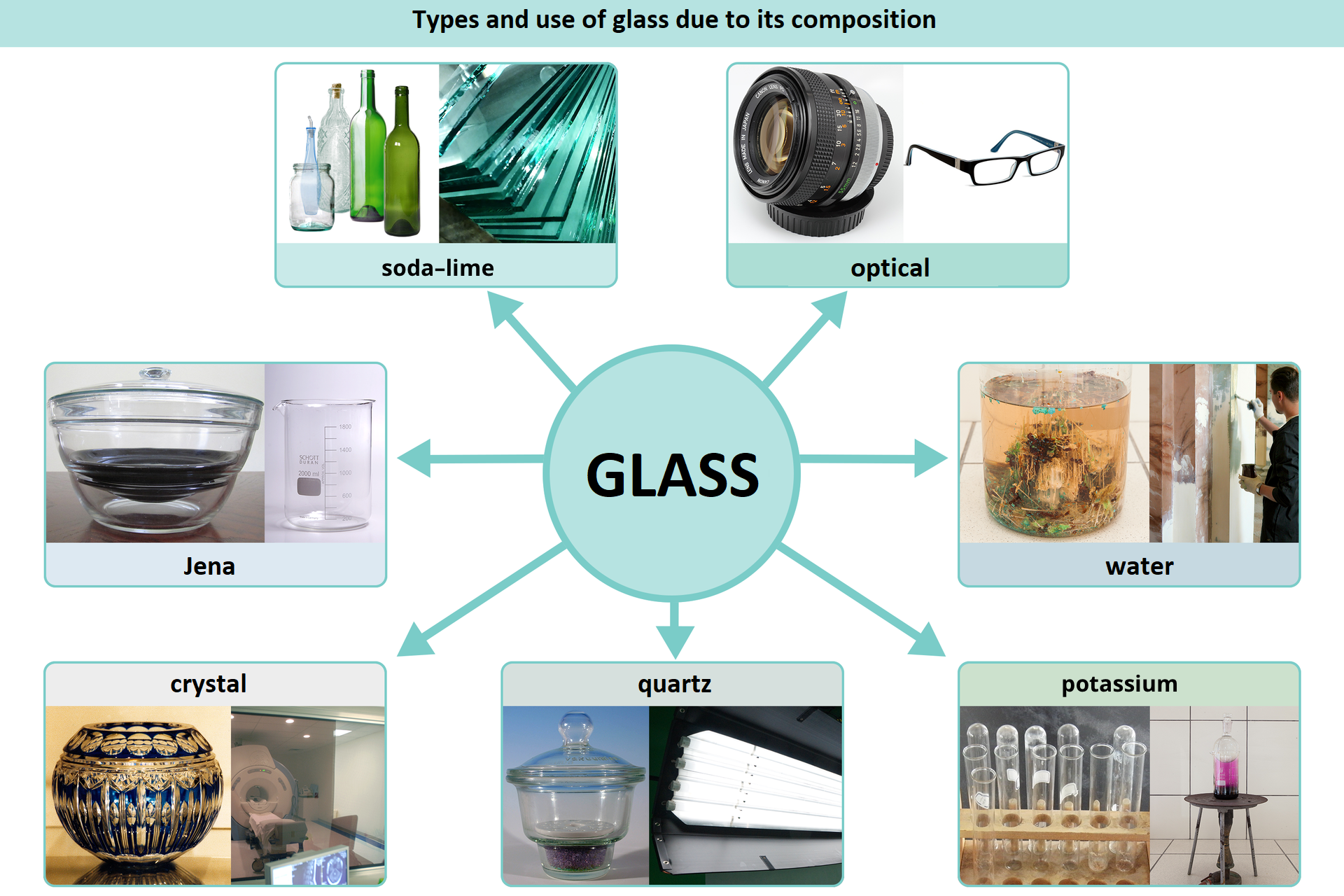
Glass is a material known and used for a long time and a modern one at the same time – with a growing future. Due to the chemical composition, we distinguish: sodium, potassium, borosilicate, lead – crystal, quartz glass. Each type of glass has specific properties and the resulting application.
Another type of crystal glass is the so‑called Czech crystal. It is sodium‑potassium glass with a higher content of potassium oxide than sodium. The main raw materials used in the production of such glass are: glass sand, sodium, potash (potassium carbonate), limestone and barium oxide. The high refractive index and high quality of this glass leads to its application in decorative dishes, chandeliers and elegant jewellery.
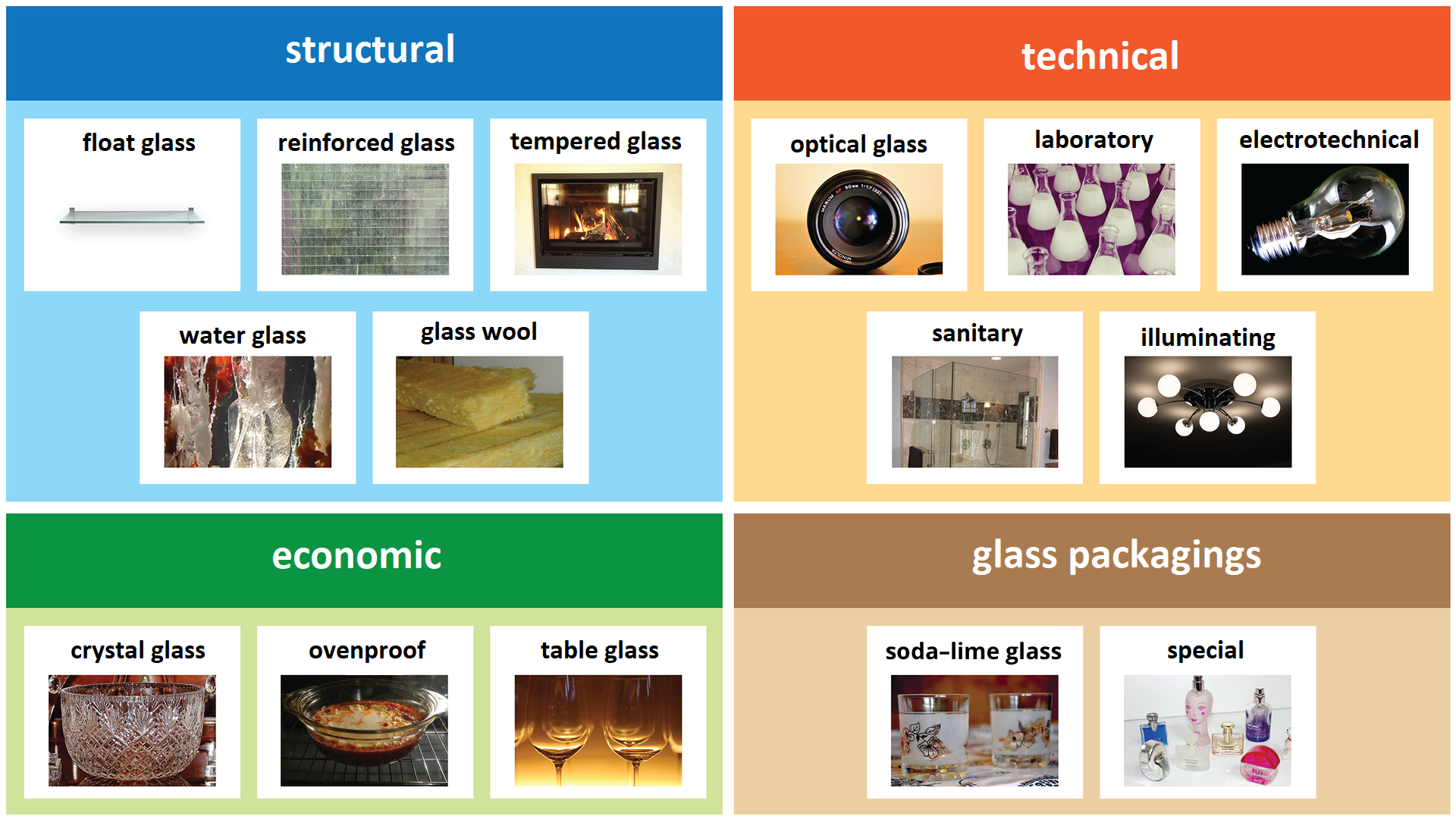
Due to the purpose, there are architectural glass (flat glass – glazing, reinforced glass, tempered glass, glass wool, water glass), technical glass (optical glass, laboratory glass, electrotechnical, sanitary, lighting), household glass (crystal, heat‑proof dishes, table glass) and glass packaging. Safety glass is: tempered glass, laminated with foil or glued, reinforced and other (chemically reinforced, foiled, fire‑resistant glazing). The dynamic development of technology in the first half of the XIX century caused the need to provide glasses for the industry characterized by high mechanical strength, chemical resistance and resistance to temperature changes, appropriate surface hardness, thermal expansion coefficient, etc. Current requirements for glass products are much greater. Among others reflective, electrically conductive, ceramic, opaque, self‑cleaning glass are produced. Products made of glass are, for example, glass blocks or glass wool. Plastic materials are strengthened with glass fibres. Then a material called a composite is created, used for the construction of car bodywork.
Self‑cleaning glass is an elevation glass equipped with a very durable titanium dioxide coating. As a result of UV radiation, the organic pollutants accumulated on the surface of the glass disintegrate and mineral contaminations lose their adhesion. 'Weakened' pollutants are easily washed away from the glass surface during rain. Self‑cleaning does not mean that the glass does not require washing at all, especially in the areas with high pollution.
Which glass contains a significant amount of lead(II) oxide and potassium oxide in its composition?
- crystal
- laboratory
- safety
- optical
In order to obtain blue glass, the following is added to the material:
- CoO
- NiO
- Cr2O3
- CaO
Potassium glass is used in the production of:
- laboratory glassware
- crystal vases
- glasses
- window panes
Summary
Glass is an amorphous material obtained by melting a mixture of sand, sodium carbonate, calcium carbonate and cullet. Additional substances that affect the glass properties are also added to the glass mass.
The wide application of glass in technology and in various areas of life is determined by its properties, such as: high hardness, transparency, low thermal and electrical conductivity, high chemical resistance, no restrictions on shape.
Due to its chemical composition, we can distinguish: sodium, potassium, borosilicate, lead, quartz and water glass.
Types of glass distinguished due to its purpose: construction, technical, economic, packaging.
Types of so‑called safety glass is: reinforced, tempered, glued, laminated and others.
Key words
glass, glass properties, quartz, raw materials of glass, cullet, amorphous body
Glossary
ciało bezpostaciowe (amorficzne) – ciało stałe, które nie ma budowy krystalicznej, charakteryzujące się chaotycznym rozmieszczeniem atomów (cząsteczek), podobnie jak w cieczach, z tą jednak różnicą, że atomy (cząsteczki) w tym przypadku nie mogą się swobodnie poruszać
izolator elektryczny – materiał, który nie przewodzi prądu elektrycznego
piszczel szklarska – narzędzie używane przez szklarza (hutnika) do formowania szkła; ma kształt długiej, dość cienkiej rurki; na jednym końcu znajduje się nabel, na który nabiera się z pieca roztopioną masę szkła; na drugim końcu jest ustnik, przez który wydmuchuje się nabraną masę
przewodnik elektryczny – materiał, który dobrze przewodzi prąd elektryczny
surowce szklarskie – piasek, soda, wapień, stłuczka szklana oraz środki barwiące
szkło – tworzywo o nieuporządkowanej strukturze, otrzymywane w wyniku schłodzenia stopionej mieszaniny piasku kwarcowego, wapienia i sody
szkło float – szkło płaskie produkowane metodą „floatowania”, czyli równomiernego „rozlewania“ masy szklanej na powierzchni stopionej cyny
wełna szklana – materiał stosowany do izolacji termicznej i akustycznej, otrzymywany w wyniku topienia w temperaturze 1000°C piasku kwarcowego, stłuczki szklanej z dodatkiem skał takich jak: dolomit, wapień; roztopiony surowiec poddaje się procesowi rozwłókniania, a do otrzymanych włókien dodaje się spoiwo (np. żywice)
zestaw szklarski – mieszanina surowców, z których powstaje masa szklarska