Higher carboxylic acids
what structure and properties lower carboxylic acids have.
what the name 'higher carboxylic acids' means;
to call higher carboxylic acids 'saturated' (palmitic and stearic acid) and 'unsaturated' (oleic acid);
to write the formulas for higher carboxylic acids (palmitic, stearic and oleic acids);
what the properties of higher carboxylic acids are;
what the application of higher carboxylic acids is;
to design an experiment allowing to distinguish between oleic acid and palmitic/stearic acid.
Higher carboxylic acids
Carboxylic acids, among others the acetic acid or butyric acid , due to the small number of carbon atoms in the hydrocarbon chain, are classified as lower carboxylic acids. Higher carboxylic acidsHigher carboxylic acids are compounds containing a long carbon chain (a dozen or so carbon atoms) as well as the group which is characteristic of carboxylic acids.
Higher carboxylic acids are also called fatty acids. Fat molecules contain radicals of fatty acids.
Division of higher carboxylic acids
Remember the concept of organic saturated and unsaturated compounds. Do you recall which hydrocarbons are classified as saturated, and which as unsaturated?
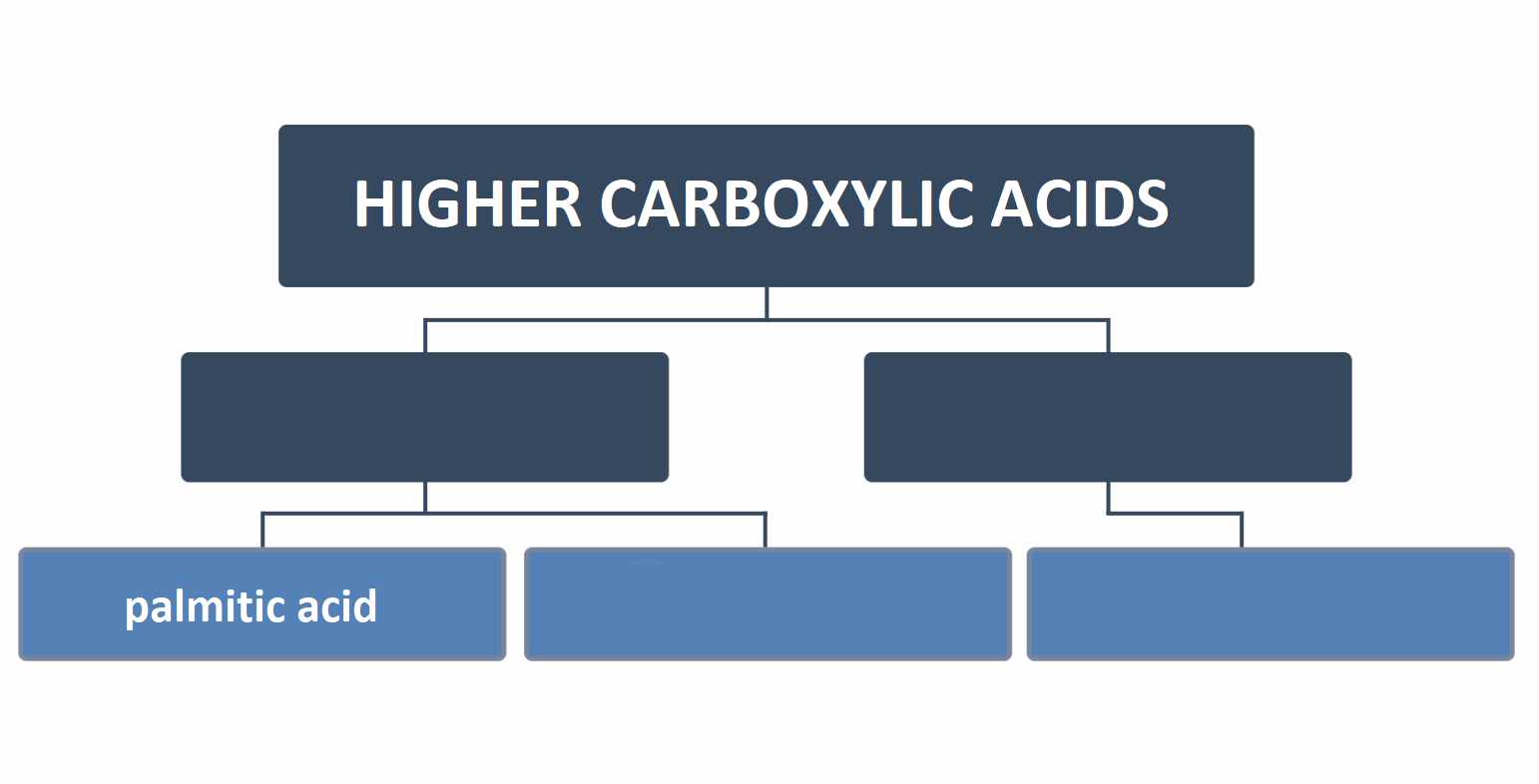
There are saturated and unsaturated fatty acids.
Saturated higher carboxylic acids include palmitic acidpalmitic acid that has the following formula: , as well as stearic acidstearic acid . In the molecules of these acids, there are single bonds between carbon atoms. Oleic acidOleic acid contains a double bond between the 9th and 10th carbon atoms, which is why it belongs to unsaturated organic compounds.
Properties of higher carboxylic acids
Do higher and lower carboxylic acids have similar properties?
Due to the long carbon chain, the properties of higher carboxylic acids differ from those of lower carboxylic acids.
test tubes,
watch glasses,
water,
acetic acid,
palmitic acid,
stearic acid,
oleic acid.
Determine the state of aggregation, color and odor of the carboxylic acids used in the experiment.
Test the solubility of acids in water.
Note the results of the observations in the form of a table:
As a result of the experiment, it was observed that higher carboxylic acids have different physical properties from lower carboxylic acids. Higher carboxylic acids do not dissolve in water due to the long carbon chain. Saturated fatty acids – palmitic and stearic acids – are white solids. Unsaturated oleic acid is an oily liquid of a slightly yellowish color; it has the characteristic odor of old oil.
Stearic and palmitic acids melt at low temperatures. Carboxylic acids undergo the combustion reaction. They burn with a yellow flame – like the one we observe while candles burn.
Arrange the signatures on the diagram to correctly represent the distribution of higher carboxylic acids.
stearic acid, saturated, oleic acid, unsaturated
Match the definitions
a saturated higher carboxylic acid with the following formula: C<sub>15</sub>H<sub>31</sub>COOH, an unsaturated higher carboxylic acid with the following formula: C<sub>17</sub>H<sub>33</sub>COOH, containing one double bond between the 9th and 10th carbon atoms, chemical compounds containing a long carbon chain and the carboxylic group –COOH; also referred to as fatty acids, a saturated higher carboxylic acid with the following formula: C<sub>17</sub>H<sub>35</sub>COOH
| higher carboxylic acids | |
|---|---|
| palmitic acid | |
| stearic acid | |
| oleic acid |
Mark the true statements.
- Higher carboxylic acids have short carbon chains.
- Stearic and palmitic acids are found in candles, soap and lard.
- Saturated acids discolor bromine water.
- Complete combustion of oleic acid produces carbon monoxide and water.
- Melted stearic acid cannot be distinguished from oleic acid.
Exercise summary
In order to distinguish stearic or palmitic acid from oleic acid, use bromine water or a solution of potassium permanganate . The molecules of unsaturated oleic acid contain a double bond and therefore discolor the bromine water and the solution of potassium permanganate. What is more, this reaction produces a white solid – saturated stearic acid. It transitions into a saturated acid, which we observe as a change in the state of the substance from liquid to solid. Higher carboxylic acids introduced into water with the addition of methyl orange do not change the color of the indicator because they do not dissolve in water and do not undergo the process of electrolytic dissociation.
How are soaps made?
Fatty acids play an important role in obtaining soaps.
Will the reaction of higher carboxylic acid with sodium hydroxide produce salt?
Stearic acid reacts with the sodium hydroxide, and the products of this reaction are salt and water.
stearic acid,
aqueous solution of sodium hydroxide,
phenolphthalein,
burner,
test tubes.
Pour a small amount of stearic acid into a test tube.
Pour in a few cubic centimeters of the aqueous solution of sodium hydroxide.
Add a few drops of phenolphthalein.
Heat the mixture.
Observe the changes.
Leave the substance to cool down.
The mixture of stearic acid and sodium hydroxide melts. The raspberry color disappears. We observe foaming of the reaction product. After cooling down, a lumpy substance with a soap‑like consistency is created.
Stearic acid reacts with the sodium hydroxide to form soap. The reaction proceeds according to the following equation:
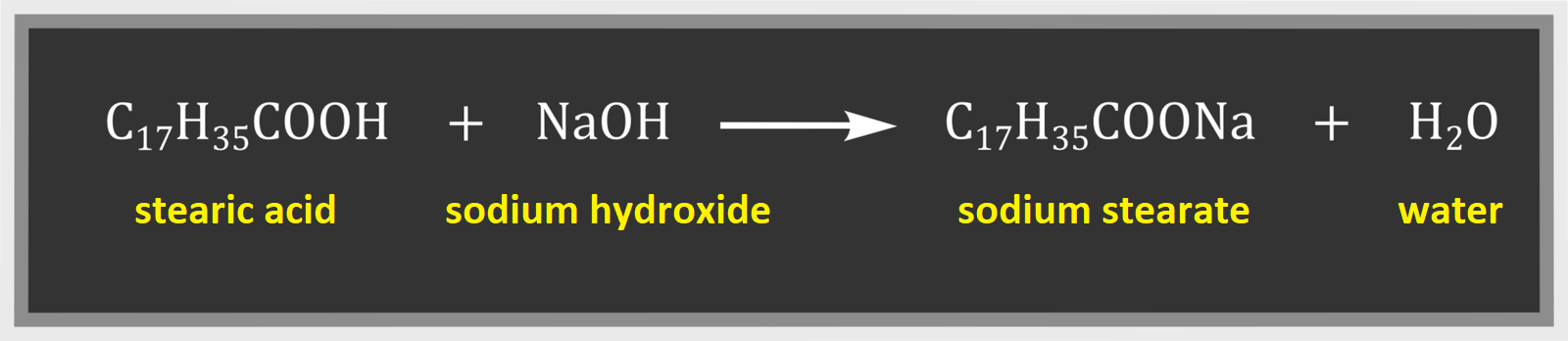
Sodium stearate is the water‑soluble sodium salt of stearic acid.
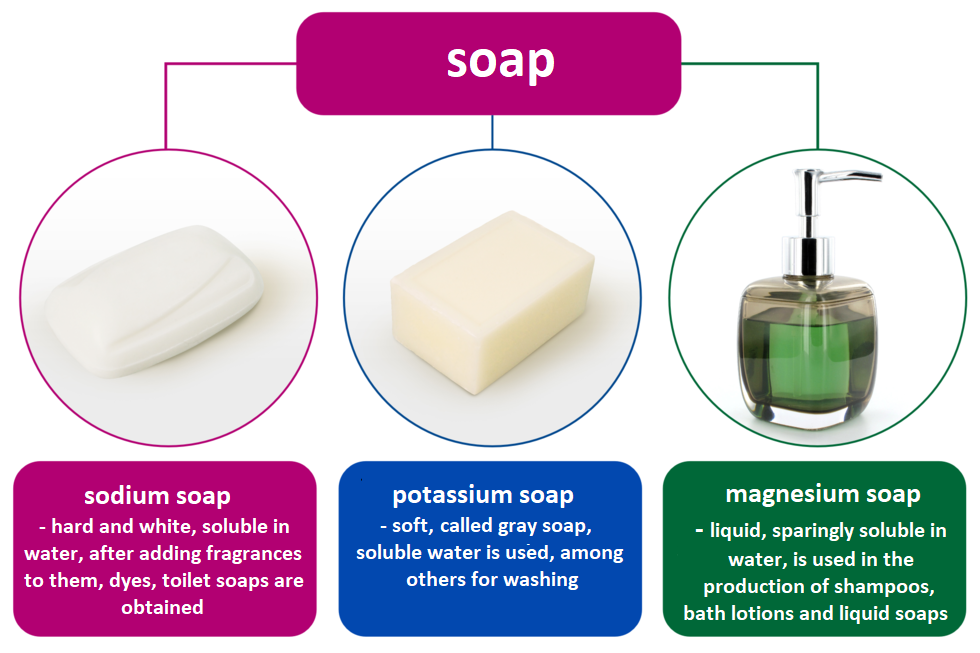
The salts of higher carboxylic acids, e.g. palmitic, stearic and oleic, are soaps.
Conclusion
Higher carboxylic acids are acids containing long carbon chains.
Higher carboxylic acids are also referred to as fatty acids because fat molecules contain the radicals of these acids.
Saturated fatty acids include palmitic and stearic acids.
Oleic acid is an unsaturated fatty acid.
Soaps are the salts of higher carboxylic acids.
Write chemical equations for complete combustion of stearin's components, i.e. palmitic and stearic acids.
Keywords
carboxylic acid, long‑chain monocarboxylic acids, molecular formula, fatty acid, palmitic acid, stearic acid, oleic acid
Glossary
wyższe kwasy karboksylowe - chemical compounds containing a long carbon chain and the carboxylic group ; also referred to as fatty acids.
kwas palmitynowy - a saturated higher carboxylic acid with the following formula:
kwas stearynowy - a saturated higher carboxylic acid with the following formula:
kwas oleinowy - an unsaturated higher carboxylic acid with the following formula: , containing one double bond between the 9th and 10th carbon atoms