Hydrates
that the salts of sulphuric acid are sulphates;
that the molecular mass of the compound is the mass of the molecule expressed in units of atomic mass [u];
that the outer layer of the earth's crust form various rocks and minerals.
to write down formulas and create hydrates nomenclature;
to interpret differences in the properties of hydrates and anhydrous substances;
to describe the hardening process of gypsum mortar and to note down the appropriate reaction equation;
to discuss the use of gypsum rock;
to justify why calcined gypsum is used in medicine.
Types of gypsum rocks
Gypsum rock is a sedimentary rock along with limestone and chalk. Their main ingredient is calcium sulphate building the minerals such as gypsum and anhydrite. Deposits of gypsum flowergypsum flower (), which is an example of a hydrate, were formed during the evaporation of saline waters of lakes and rivers at a temperature lower than 42°C. Gypsum flower can also create a variant called alabaster (). However, anhydrite crystallizes at higher temperatures (c), anhydrous calcium sulphate, Latin an – without and hydro – water, so‑called anhydrous gypsum.

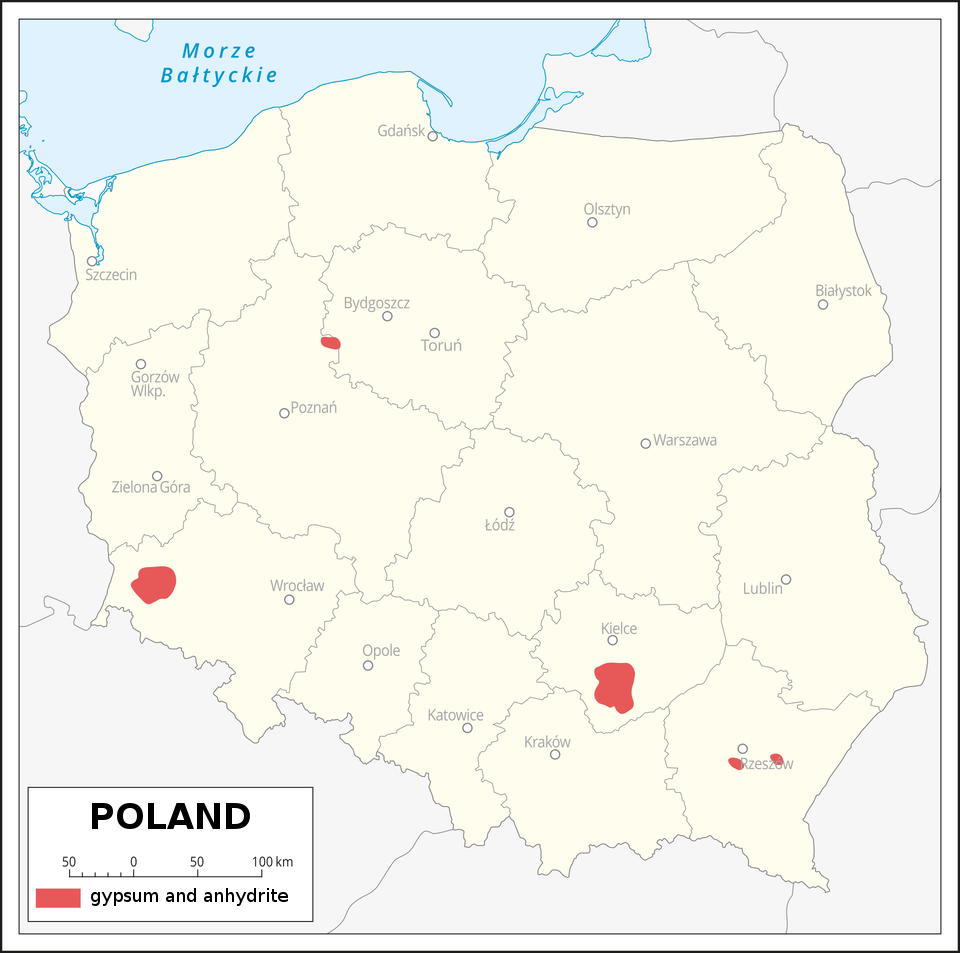
There are generous deposits of gypsum and anhydrite in Poland. The largest deposits are found in i.e. the valley of the Nida River (these deposits are among the largest in Europe), in Niwnice near Lwówek Śląski, in the Głogów glacial valley, on the edge of Świętokrzyskie Mountains.
Gypsum flower creates beautiful crystals in various colours: red, grey, white, but most often it is colourless in natural conditions. It has a characteristic silky, pearly or glassy gloss. Its hardness in the Mohs scale of mineral hardness is 2, so it is soft and fragile mineral. The density of this mineral is approx. 2.3 g/cmIndeks górny 33.
Alabaster is a translucent, white or slightly coloured material, e.g. yellowish, greenish, pinkish. Gypsum alabaster can be easily scratched with a fingernail. In the natural conditions calcite alabaster can be found. The hardness distinguishes it from gypsum alabaster because it is much more difficult to be scratched – it can be done only with a sharp knife of good quality steel. The range of density of calcite alabaster is 2.7–2.8 g/cmIndeks górny 33.
Anhydrite can also be found in various colours: white, grey, blue or colourless. It always is transparent with a characteristic glassy or pearly gloss. Its Mohs hardness is 3–3.5, and the density is about 2.9–3.0 g/cmIndeks górny 33.
Desert rose (sandy rose, crystal rose) is a crystal clusters of gypsum resembling the appearance of rose petals, due to which it has great decorative qualities. In addition to the predominant amount of gypsum, it also contains various amounts of quartz sand. It is formed in desert areas, in conditions of dry and hot climate, due to the evaporation of strongly mineralized salt lakes or ground waters, which crystallize in the near‑surface layer of loose sand in a rose‑form.

Structure and nomenclature of hydrates
HydratesHydrates are hydrated salts. These are chemical compounds that contain water molecules built into the crystal structure. Water contained in hydrates is called water of crystallization or hydration. There are five molecules of water for one copper(II) cation and one sulphate anion in the copper(II) sulphate pentahydrate. Often, the same substance may form several different hydrates, for example sodium carbonate (, ).
The symbolic notation of the presence of water in hydrates is a dot, which does not represent the sign of multiplication, but indicates the presence of water in the crystal structure. The hydrate names are created in several ways. The systematic name recommended by the PTCh Nomenclature Commission (based on IUPAC recommendation) includes: the name of anhydrous salt, a long line, the word „water” and mutual proportions of ingredients in this compound contained in brackets.

Hydrates properties
Before watching the movie “Heating the gypsum flower” write down the research question and hypotheses. During the screening, pay attention to what happens to the gypsum flower during heating, and then note observations and conclusions from the experiment.

Film dostępny na portalu epodreczniki.pl
Film video przedstawiający wykrywanie wody w gipsie krystalicznym. Do wykonania eksperymentu służą: burner, peteri dish with crystal gypsum, spatula, test tube, retort stand. Laborant w rękawiczkach ochronnych podgrzewa gips krystaliczny w probówce tak długo, aż na ściankach probówki pojawi się woda.
How to detect water in a gypsum flower?
Heating hydrates leads to the removal of the contained water.
crushed gypsum flower,
test tube,
burner,
tripod,
connecting element,
test tube clamp.
Pour the finely crushed gypsum flower into the test tube.
Place the tube onto a tripod, preferably horizontally, and heat in the flame of the burner.
Observe the changes.
As a result of heating the gypsum flower in the test tube, a white fine‑grained solid is formed, and droplets of a colourless liquid appear on the walls. There is a transformation in the system that can be described by the equation:
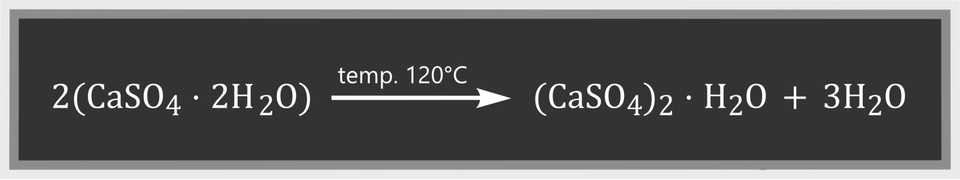
The products of this reaction are calcined gypsum and water. The temperature at which this reaction takes place is about 120°C. At temperatures above 180°C, the gypsum flower loses water completely and forms anhydrous calcium sulphate:
What is the systematic name of calcined gypsum?
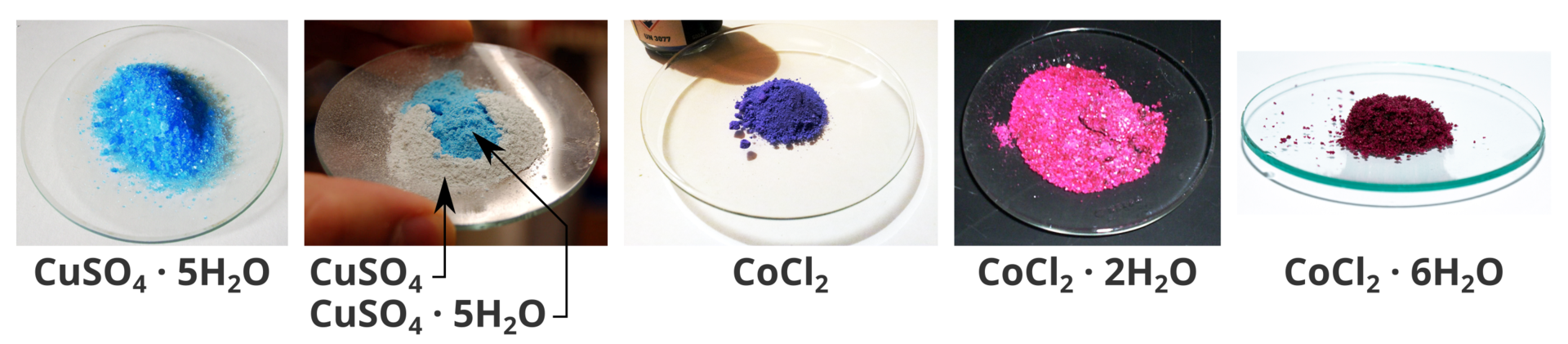
Some anhydrous salts may differ from their hydrates by colour. An example may be the so‑called bluestone, i.e. [copper(II) sulphate–water (1/5)], which is formed from blue crystals. While heating it, the blue colour gradually turns white. The salt used to detect water behaves in a similar way because it changes its colour in a characteristic way depending on the number of molecules of water of crystallization, i.e. the anhydrous cobalt(II) chloride is blue, dihydrate – pink and hexahydrate – red.
Summary
Gypsum rock contains calcium sulphate.
The calcined gypsum is formed as a result of gypsum flower’s calcinating in the 120°C. When the temperature is higher than 180°C, the anhydrous calcium sulphate is formed.
Gypsum flower and calcined gypsum are examples of hydrated salts (hydrates). Anhydrite is an example of anhydrous salt.
Hydrates are unstable and, during heating, are transformed into anhydrous salts or salts with lower degree of hydration.
Gypsum mortar is an example of hydraulic lime mortar.
Gypsum rock is used in construction, medicine, agriculture and in the ceramic, food, chemical industry. It is also a valuable material in the hands of artists.
Keywords
gypsum, calcined gypsum, anhydrite, calcium sulphate, hydrates, gypsum mortar
Match the pairs: English words with Polish definition.
siarczan(VI) wapnia---woda(2/1), siarczan(VI) wapnia---woda(1/2), w chemii nieorganicznej, sole, które zawierają cząsteczki wody wbudowane w sieć krystaliczną, mieszanina gipsu palonego i wody, twardniejąca pod wpływem wiązania wody, cząsteczki wody wbudowane w sieć krystaliczną hydratów
| hydrates | |
| water of crystallization (water of hydration) | |
| gypsum flower | |
| calcined gypsum | |
| gypsum mortar |
Glossary
hydraty – w chemii nieorganicznej, sole, które zawierają cząsteczki wody wbudowane w sieć krystaliczną
woda krystalizacyjna (hydratacyjna) – cząsteczki wody wbudowane w sieć krystaliczną hydratów
gips krystaliczny – siarczan(VI) wapnia–woda(1/2)
gips palony – siarczan(VI) wapnia–woda(2/1)
zaprawa gipsowa – mieszanina gipsu palonego i wody, twardniejąca pod wpływem wiązania wody