Metal alloy
what are the properties of matter in different states of matter;
what are symbols of chemical elements and how to use them;
what metals are and what properties they have;
what are applications of metals in everyday life;
which properties of metals allow to differentiate them;
according to which criteria mixtures are classified;
what safety rules should be followed in the school chemical laboratory.
to explain why instead of metal, its alloy combined with other chemical elements is often used;
to name examples of applications of metal alloys in everyday life;
in the periodic table of elements to indicate metals from which utility alloys are obtained;
to classify metal alloys as homogeneous mixtures and describe some of them: bronze, brass, steel, duralumin;
to design experiments to compare the properties of metals and their alloys.
What music and metal alloys have in common?
Consider and answer following questions:
What music and chemistry can have in common?
What materials are musical instruments made of?
Note down your answers.
While listening to the organ recital, it is worth considering what the most important elements of the organs – pipes – are made of? Most often it is a tin and lead alloy. The sheet of metal building the pipes may have different tin contents, and the percentage of this element in the alloy is determined by the so‑called tin‑assay. It can be value from 1 to 16. Tin‑assay in subsequent samples can be calculated by multiplying 6.25% by assay “number” (assay 1 contains 6.25% of tin, 2 - 12.50% of tin, 3 - 18.75% of tin, etc.). Assay 16 means there is no lead added, only tin (100%) is included in this material. It is rarely used and it has mainly decorative qualities. The higher the tin content, the lower the density and the lighter the colour of the alloy. However, the more lead, the greater the density of the alloy, and the colour is darker.

In the lower part of the organ pipes, the walls are often thicker than at the top. Due to this, these withstand the high pressure of the pipe body on its lower part, which prevents its deformation (flattening). Sometimes large pipes are made of zinc - lighter and cheaper than lead, however, then the emitted sound has lower quality and their surface is dull and does not have gloss, as zinc in the air undergoes passivation. Brass instruments, such as trombone or trumpet, are most often made of brassbrass, i.e. copper and zinc alloy, rarely of noble metal. Alto flutes can be produced from the so‑called gold brass (with a high content of copper), due to which these are lighter than traditional instruments and have optimal sound.
Guitar manufacturers also use metal alloys. They are constantly looking for new ways to make the strings of these instruments last longer and have better sound qualities. Most of the currently produced strings for electric guitars are made of nickel steel, ensuring good sound quality. The current news are strings made of stainless steel – very strong, durable and hard.
Why are the guitar strings coated with plastics? Formerly the guitar strings were produced from processed intestines of animals, nowadays nylon strings are used most often in classical guitars, and in the rest of them – string made of steel or bronzebronze. Dirt, sweat and grease covering the strings while playing expose them to corrosion.
Therefore, these are protected against impurities by covering them with plastic coatings.
What are metal alloys and how to obtain these?
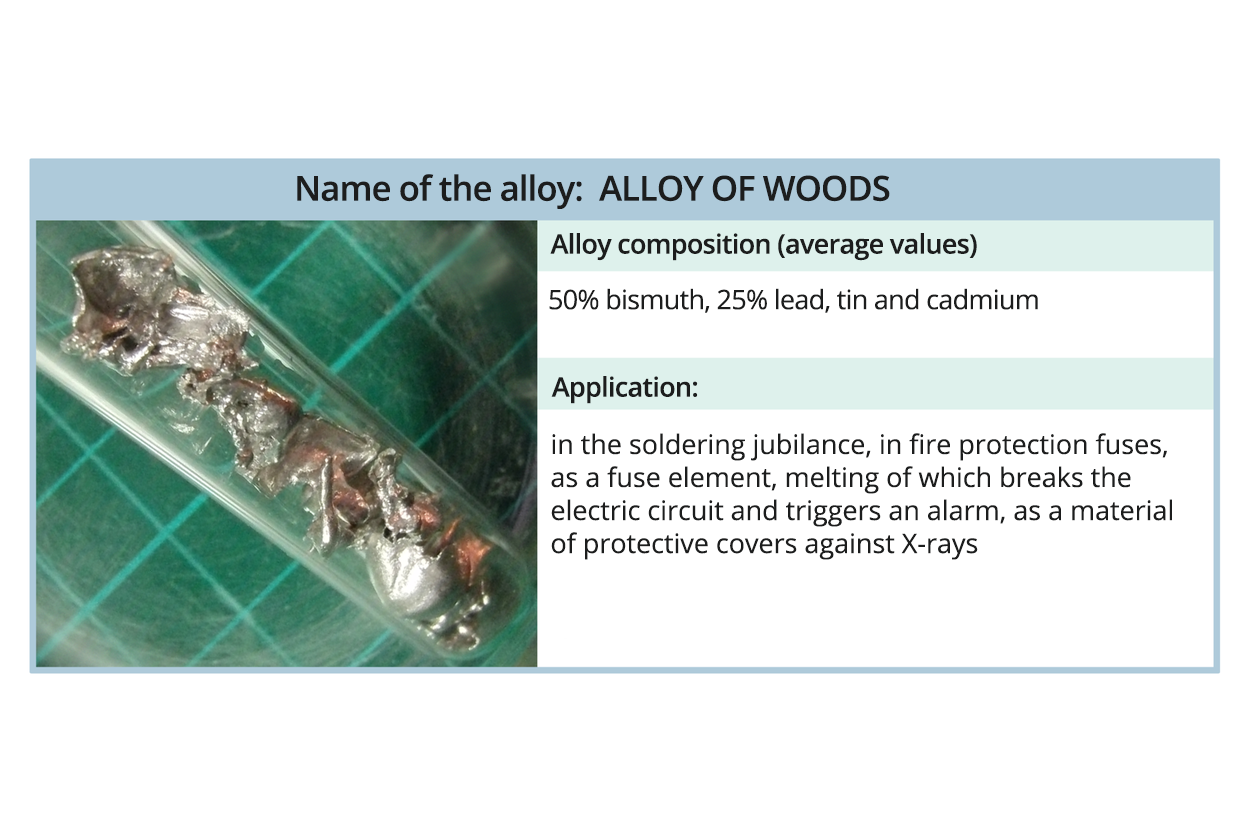
Before you watch the movie „Obtaining Wood's alloy”, write down the research question and hypotheses. After the screening, make notes of observations and conclusions.

Film dostępny na portalu epodreczniki.pl
Film pokazuje otrzymywanie stopu Wooda. Będziesz potrzebować: bizmutu, cyny, ołowiu, kadmu, kalafonii, statywu, trójkąta kaolinowego, tygla żelaznego lub porcelanowego, pręta żelaznego, wagi, palnika gazowego, rury z tektury. Instrukcja: Umieść 5 g cyny w tyglu i dodaj kalafonię. Podgrzej tygiel, aż cyna się roztopi, a następnie, przy stałym ogrzewaniu, dodaj kolejno: 10 g ołowiu, 5 g kadmu i 20 g bizmutu. Zmieszać zawartość tygla za pomocą żelaznego pręta do uzyskania jednorodnej mieszaniny. Pozwolić na stopienie uzyskanego stopu, usunąć go z tygla i usunąć pozostałą kalafonię. Można go ponownie stopić i wylać z niego pręty za pomocą kartonowej rury.
Do the alloys have the same colour and gloss as the metals these were made of?
Choose one of the presented hypotheses, and then verify it.
The alloy has the same colour and gloss as the metals this is made of. The alloy has different colour and gloss as the metals this is made of.
bismuth,
tin,
lead,
cadmium,
rosin,
tripod,
kaolin triangle,
an iron or porcelain crucible,
iron rod,
scale,
gas burner,
tube made of cardboard.
Place 5 g of tin in the crucible and add rosin.
Heat the crucible until the tin melted, and then, with constant heating, add in order: 10 g of lead, 5 g of cadmium and 20 g of bismuth.
Mix the contents of the crucible with an iron rod until a homogeneous mixture is obtained.
Allow the obtained alloy to solidify, remove it from the crucible and remove the remaining rosin.
You can melt it again and cast rods from it using a cardboard tube.
As a result of the melting of two or more different metals homogenous mixtureshomogenous mixtures called alloys are obtained. Both metals and alloys obtained from these have metallic gloss.
AlloyAlloy i.e. a mixture of metals or metal with non‑metal elements, it is obtained by melting the components and then cooling the obtained mass.
Properties of metal alloys
Find following elements in the periodic table: tin, lead, copper, zinc.
Write down which alloy is created by given metal pairs: tin and lead, copper and tin, copper and zinc. Compare answers with the Metal Alloys table.
Using the periodic table of elements and chemical tables, compare the melting points of individual metals and their alloys.
Analyse the information on the illustration below and recall the history of the stone, bronze and iron ages. Make a short note about the metals and objects made of them used in these eras.

1. Stone age
2. Bronze age
3. Iron age
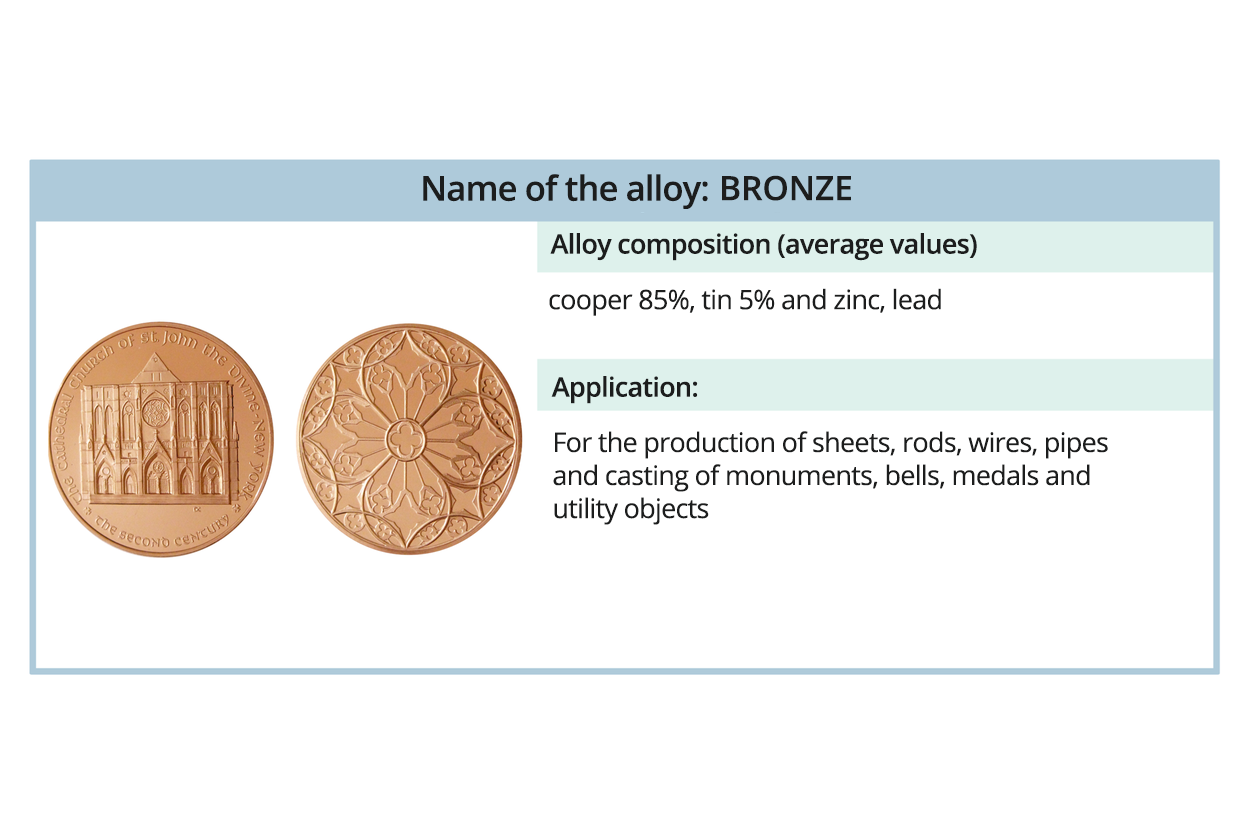
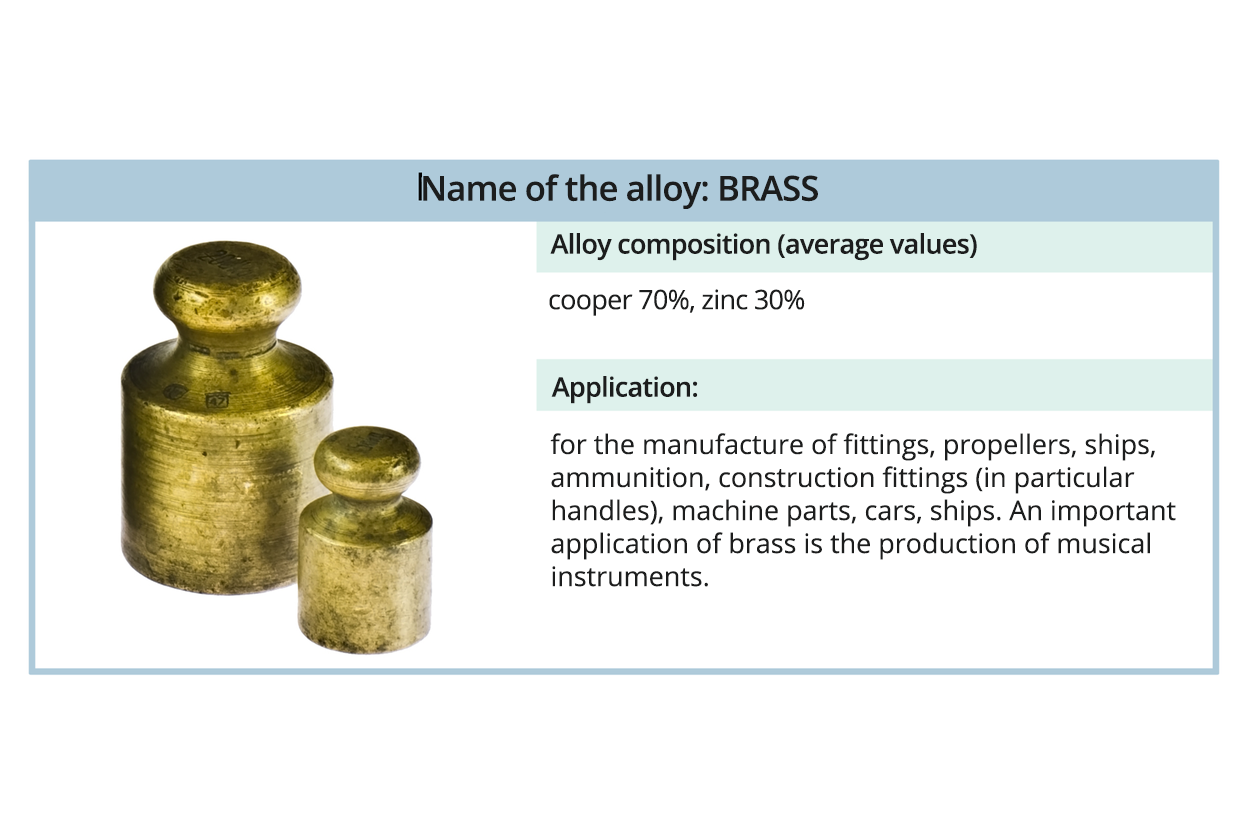
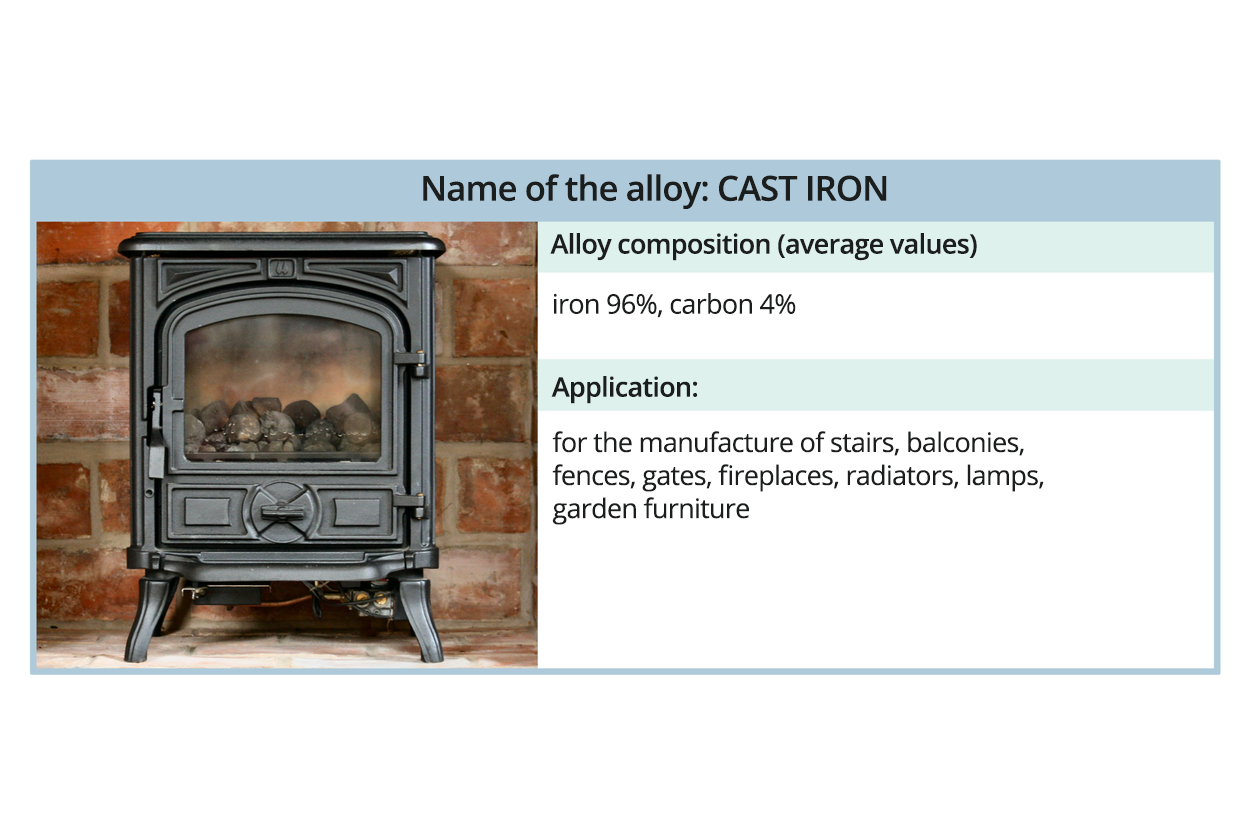
Watch the gallery presenting metal alloys. Memorise what their applications are.
Iron due to its properties is the most useful metal. It is the main component of commonly used steel, which is obtained from crude steel (produced in a huge furnace from iron ore) and refined with metals: manganese, nickel or chromium. This alloy is used in the construction of bridges, skyscrapers, ships, offshore platforms, cars, trains, roofs or in the manufacture of cans. For manufacturing, among others elements of the construction of aircraft or beverage cans, another alloy is used – duraluminduralumin.
Rust‑coloured alloy
This alloy was first obtained in the United States during the Great Depression (1929 – 1933). It is a material valued due to its special properties, thanks to which it does not require frequent maintenance. Corten (Weathering steel), a steel grade, was patented in 1933. The material name is derived from its corrosion resistant and high tensile strength properties. For its production, among others chromium, copper, silicon and phosphorus were used. The alloy, under the influence of atmospheric conditions, is covered with a thin protective coating reminiscing brown rust. This makes the steel corrosion‑resistant, durable and resistant to tension.

Do you think that metal alloys have the same properties as the components that create them? Write down the answer. Suggest a method for testing differences in the physical properties of several substances and their mixtures, e.g. metals and their alloys.
Before you watch the movie „Testing the properties of the alloy and its components”, write down the research question and hypotheses. After the screening, make notes of observations and conclusions.

Film dostępny na portalu epodreczniki.pl
Film przedstawia eksperyment. Właściwości stopu Wooda. Materiały wykorzystane w eksperymencie: stop Wooda, termometr, zlewka z wodą. Do wody należy dodać stop Wooda, a następnie zmierzyć temperaturę. Temperatura wynosi siedemdziesiąt jeden stopni Celsjusza. Stop się topi.
Alloy usually has different properties from its building blocks, in some cases even a small number of additives significantly affects its properties. Typically, the alloys are more resistant to air and water, as well as harder and more mechanically durable than pure metals.
Solve the crossword and explain the meaning of the clue.
- Copper and zinc alloy
- The most popular metal alloy
- Alloys are obtained after fusing two or more metals together and ... the obtained mass.
- The phenomenon of destruction of metals and their alloys
- High amount of lead in alloy increases its ...
| 1 | |||||||||||
| 2 | |||||||||||
| 3 | |||||||||||
| 4 | |||||||||||
| 5 |
Summary
The properties of the alloy depend on its composition and the method of its manufacture.
Metal alloys are obtained just as other mixtures by mixing two or more metals, after fusing them together and cooling the obtained mass.
The alloys differ from the metals creating them with their properties, e.g. hardness, durability, ductility and melting points (generally lower in the case of alloy). Metal alloys are more resistant to corrosion than metals.
Steel is an iron alloy with an admixture of carbon, which provides greater hardness and durability of the alloy and reduces its forgeability and ductility. Achieving specific properties, e.g. corrosion resistance, requires the addition of chromium to the steel, e.g. stainless steel contains 11 – 14% of chromium. Steel prepared in this way is used for the manufacture of machine parts, rails, knife blades, tools, concrete reinforcements, building elements.
Brass is an alloy of copper and zinc (up to 40%) and additions, e.g. lead, aluminium, tin, manganese, iron, chromium and silicon. Brass is useful for cold forming, for example during the production of ammunition shell‑case. Coins, medals, candlesticks, cups, padlocks, mortars, monuments, decorative elements (buckles, door handles, weights, bells, fittings, picture frames), fixtures, equipment resistant to seawater, screw propellers, ammunition are made of brass. Elements of the machines are also made of brass – in the machine, automotive, electrotechnical, shipbuilding, precision and chemical industries. An important application of brass is the production of musical instruments.
Prepare an infographic, a presentation or speech illustrating the preparation and application of a selected metal alloy.
Key words
metal alloys, bronze, brass, duralumin, steel, cast iron, Wood’s alloy
Match the pairs: English words with Polish definition.
mieszanina, w której składników nie możemy rozróżnić wzrokiem ani za pomocą prostych przyrządów optycznych, stop miedzi i cynku, mieszanina jednorodna metali (np. brąz, mosiądz, cyna do lutowania) lub metalu z dodatkiem metali i niemetali (stal), uzyskiwana przez stopienie składników, a następnie schłodzenie otrzymanej masy, stop miedzi i cyny, stop glinu, zawiera zwykle miedź, mangan i magnez, stop żelaza z węglem i dodatkami innych pierwiastków, np. chromu, niklu, manganu, krzemu
| bronze | |
| duralumin | |
| homogenous | |
| brass | |
| steel | |
| alloy |
Glossary
brąz – stop miedzi i cyny
duraluminium – stop glinu, zawiera zwykle miedź, mangan i magnez
mieszanina jednorodna – mieszanina, w której składników nie możemy rozróżnić wzrokiem ani za pomocą prostych przyrządów optycznych
mosiądz – stop miedzi i cynku
stal – stop żelaza z węglem i dodatkami innych pierwiastków, np. chromu, niklu, manganu, krzemu
stop – mieszanina jednorodna metali (np. brąz, mosiądz, cyna do lutowania) lub metalu z dodatkiem metali i niemetali (stal), uzyskiwana przez stopienie składników, a następnie schłodzenie otrzymanej masy