Physics of atom - summary
Podsumowanie wiadomości z fizyki atomu
explain the main ideas of physics of atom.
Before you start, answer the questions.
How do we describe the thermal radiationradiation of bodies?
Explain the concepts of continuous, line, emissionemission and absorptionabsorption spectra.
Explain the concept of photonphoton.
Describe the atom models known to you.
What is the photoelectric effectphotoelectric effect?
1. How do we describe the thermal radiation of bodies?
Bodies at a temperature higher than absolute zero (0 K = -273⁰C) are a source of electromagnetic radiationradiation, called thermal radiation. These bodies can emit and absorb electromagnetic radiation.
The ability of bodies to emit and absorb radiation allows us to explain the existence of colours. The colour of the body depends both on the spectrum of the incident electromagnetic wave and on which wavelengths are absorbed better or worse by a given body. If the blue light is incident on a body which almost completely absorbs it, then the colour detected by the human eye would be black.
To describe thermal radiation, its emissionemission and absorptionabsorption by a body, a black bodyblack body model was created. A black body is a body that completely absorbs the electromagnetic radiation that is incident on it, regardless of the wavelength and temperature at which this process takes place.
The heated bodies emit thermal energy in the form of electromagnetic waves.
The total energy emitted by a body having a temperature T is described by the formula E = σ · TIndeks górny 44, if the ambient temperature is 0 K (where σ is a constant whose value is equal to 5,67 · 10Indeks górny -8-8). This dependence is known as the Stefan‑Boltzmann law. This formula shows that the energy emitted increases rapidly with increasing temperature.
Wien’s displacement law states that objects of different temperature emit spectra that peak at different wavelengths.
where:
b – Wien’s displacement constant, b = 2,89 · 10Indeks górny -3-3 mK,
T – body temperature expressed in absolute scale (K).
2. Continuous, line, emissionemission and absorptionabsorption spectra.
The spectrum is a registered image of electromagnetic radiationradiation. This image consists of different wavelengths (colours). Each wavelength is associated with the corresponding frequency and energy.
Instruments used for imaging and examining of spectra are spectroscopes and spectrometers.
The thermal radiation spectrum of solids and liquids is continuous - in this spectrum all wavelengths are present and there are no gaps between them; an example of a continuous spectrum is a rainbow.
The spectrum, which consists of many separate coloured lines, is called the line spectrumline spectrum.
The line spectrum is typical for gases consisting of atoms or molecules. Examples are hydrogen, helium, neon, argon and mercury or sodium vapours. All elements in the gaseous state have a characteristic line spectrum.
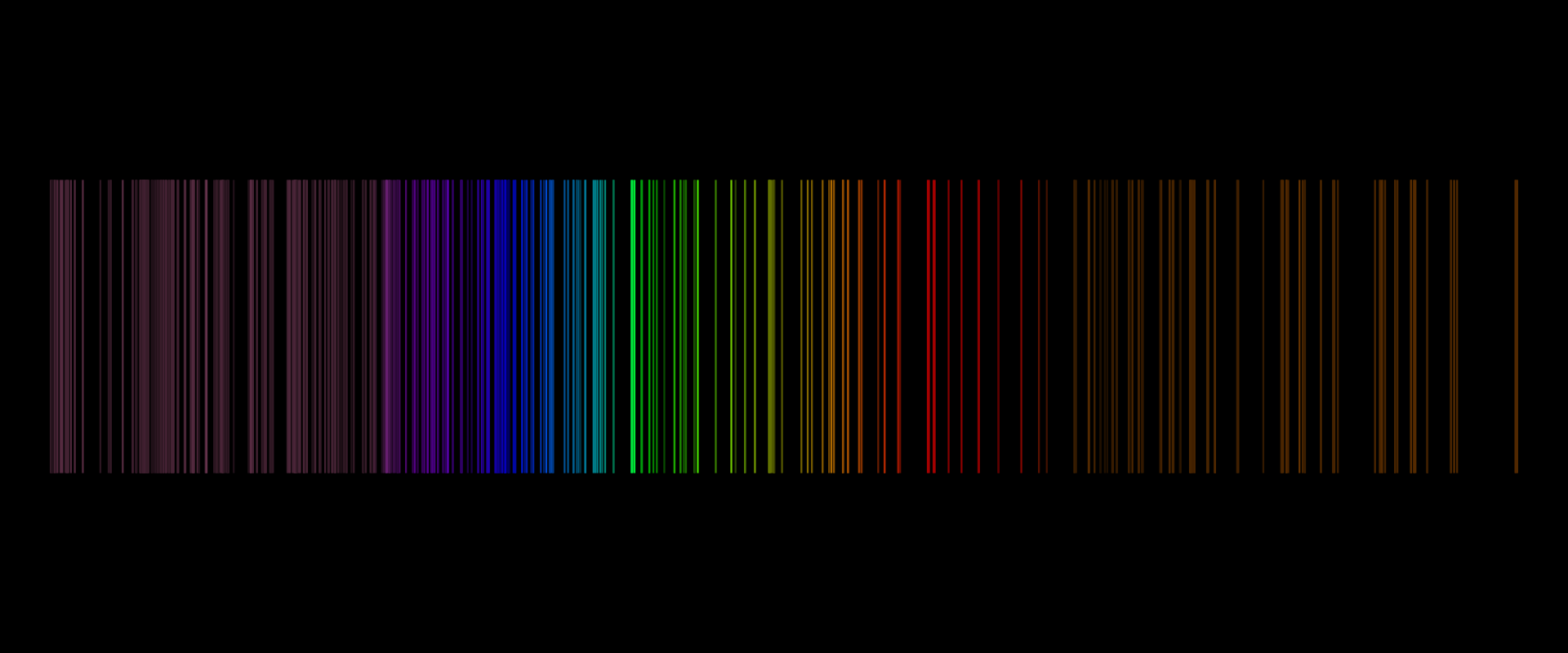
EmissionEmission spectra are spectra of radiationradiation emitted by bodies excited to glow. Line emission spectrum is generated by heated gases and continuous emission spectrum - by hot solids. Gases, whose molecules have a complex, multi‑atom structure, emit band emission spectra.
The absorptionabsorption spectrum is created as a result of absorption of electromagnetic radiation by a body.
If the radiation having a continuous spectrum passes through a cooled gas, then the energy of electromagnetic waves is absorbed exactly at the wavelengths that a given atom can emit.
In the absorption spectrum, dark lines are visible - they are located at the wavelengths that have been absorbed by a given gas.
Fraunhofer was the first to observe such dark lines in the spectrum of sunlight. We call them Fraunhofer lines.
3. What is a photonphoton?
A photonphoton is a portion (quantum) of electromagnetic radiationradiation energy. We can treat it as a particle that has the following characteristics.
- There is no rest mass.
- There is no electric charge.
- It has energy that is expressed by the formula:
where:
h – a universal constant, called the Planck constant, which is equal to 6,63 · 10Indeks górny -34-34 Js,
ν – the frequency of radiation emitted or absorbed,
c – speed of light,
λ – wavelength of radiation.
4. Atom models.
The atom's model has evolved over time. The subsequent scientific discoveries made it possible to explain the structure of the atom more and more accurately.
Thomson's model
In 1897 Thomson discovered the electron. The electron is a component of all atoms. Atom has a structure that includes electrons. This structure was called the „raisin cake” model.
The Rutherford model
The atom consists of a nucleus and electrons orbiting around it. The atom is electrically neutral (the nucleus has a positive charge, and the electrons are negative). The Coulomb force is responsible for the interaction between the atomic nucleus and its electrons. The size of the nucleus is 100000 times smaller than the size of the atom.
Bohr's model
Bohr created the atom model based on the Rutherford model. He formulated two postulates.
I. The electron can circulate around the nucleus only on selected orbits, called stationary orbits.
II. The change of atomic energy occurs only during the electron transition between stationary orbits - the transition from the higher orbit to a lower one corresponds to the energy emissionemission, and the transition from a lower to higher orbit is caused by the absorptionabsorption of energy. Energy is emitted and absorbed by the atom in the form of a portion (quantum) of energy of the value resulting from the formula:
where:
n, k - are the numbers of orbits between which the electron jumps.
With the Bohr model, the spectral line of the hydrogen atom can be explained.
The Bohr atom model allows describing precisely the structure of only a hydrogen atom; it fails when atoms have a more complex atomic nucleus, around which more electrons orbit.
Thanks to the Bohr model, the foundations of a new branch of modern physics - quantum mechanicsquantum mechanics - were established.
5. What is the photoelectric effect?photoelectric effect?
The external photoelectric effectphotoelectric effect (photoemission, photo effect) is the emissionemission of electrons from the metal surface under the influence of radiationradiation incident on this surface. These electrons are called photoelectrons.
The external photoelectric effect occurs only under certain conditions.
For each metal there is a threshold frequency (wavelength) of radiation, below (and in the case of wavelength - above) which this phenomenon does not occur at all.
The kinetic energy of emitted electrons does not depend on the intensity of radiation, but only on its wavelength.
The number of photoelectrons is proportional to the intensity of incident radiation.
The external photoelectric effect is evidence that the electromagnetic wave can be treated as a stream of particles - photons.
Photo effect is a quantum phenomenon; it became the basis of the quantum theory of light. Therefore, classical physics could not explain the photoelectric effect. In 1905 Albert Einstein explained the photoelectric effect by assuming that light is a stream of particles or photons, and one photonphoton incident on metal can transfer energy to only one electron in metal.
The principle of energy conservationenergy conservation in the interaction of photon - electron has been written in the equationequation, called the Einstein‑Millikan equation:
This equationequation says that the energy of the incident photon in the photoelectric effectphotoelectric effect is equal to the sum of the work function and kinetic energy of the electron. The work function is the minimum energy needed for the electron to leave the metal; its relationship to the threshold frequency (wavelength) has the form:
Remember
Physics of atom, or atomic physics, is a branch of physics dealing with the atom as an isolated system, consisting of the atomic nucleus and electrons orbiting around it.
Exercises
Determine which sentences are true.
- The Stefan-Boltzmann law describes the energy radiated by a black body depending on its temperature.
- Wien’s displacement law determines at what wavelength the intensity of radiation emitted by a black body reaches its minimum.
- Atom can absorb or emit radiation in the form of energy quanta called photons.
- The hydrogen spectrum is particularly important in astronomy, because the Universe consists mainly of hydrogen.
- The Balmer series lies in the infrared part of the electromagnetic spectrum.
- In the Bohr model, neutrons and protons occupy the central part of the atom called the nucleus which electrons are orbiting.
- The lowest energy state is called the ground state.
- The transition between the orbits of an atom is allowed when the absorbed or emitted energy is equal to the energy difference between these orbits.
- Emission or ejecting of electrons from the surface of the metal by radiation incident on it is called the photoelectric effect.
- The kinetic energy of emitted electrons in the photoelectric effect depends on the intensity of incident radiation.
- The photoelectric effect is evidence of the wave-particle dual nature of electromagnetic radiation.
- Albert Einstein received the Nobel Prize in physics in 1921 for explaining the photoelectric effect.
Search in the available sources for information how a photocell works. Write a note in the notebook.
Explain in English what the Balmer series is.
Indicate which pairs of expressions or words are translated correctly.
- absorpcja - radiation
- promieniowanie - absorption
- ciało doskonale czarne - black body
- foton - photon
- widmo liniowe - line spectrum
- efekt fotoelektryczny - photoelectric effect
- ciało doskonale czarne
- radiation
- line spectrum
- black body
- widmo liniowe
- foton
- photoelectric effect
- photon
- efekt fotoelektryczny
- promieniowanie
Glossary
absorpcja
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: absorption
ciało doskonale czarne
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: black body
emisja
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: emission
zachowanie energii
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: energy conservation
równanie
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: equation
widmo liniowe
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: line spectrum
efekt fotoelektryczny
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: photoelectric effect
foton
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: photon
mechanika kwantowa
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: quantum mechanics
promieniowanie
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: radiation
Keywords
black bodyblack body
line spectrumline spectrum
photoelectric effectphotoelectric effect
photonphoton
radiationradiation