Preparation, properties and application of carbon dioxide
how can you prove that air exists;
what is the air and what is its composition;
how can you prove that the air is the mixture by the experiment;
which criteria are adopted for the classification of types of chemical reactions;
how equations of chemical reactions are recorded;
what are oxides and how these can be obtained by combustion;
to name the sources of carbon dioxide;
to write down the molecular and structural formula of carbon dioxide;
to plan and conduct experiment allowing to obtain and identify carbon dioxide;
to design and conduct experiments to examine the basic properties of carbon dioxide.
Obtaining carbon dioxide and its properties
Watch the movie. Note which chemical compound was obtained and how? Which properties of this compound justify what has happened to the candle?

Film dostępny na portalu epodreczniki.pl
Film pokazuje eksperyment. Problem badawczy: Czy dwutlenek węgla otrzymany z sody oczyszczonej i octu podtrzymuje palenie się świeczki? Hipotezy: Dwutlenek węgla jest gazem, który podtrzymuje palenie się świeczki. Dwutlenek węgla jest gazem, który nie podtrzymuje palenia się świeczki. Co będzie potrzebne: plastikowa butelka, soda oczyszczona, ocet, balon, świeczka typu podgrzewacz, dwie zlewki, lejek. Instrukcja: Do butelki wlej niewielką ilość octu (maksymalnie jedną trzecią objętości butelki). Do balonu za pomocą lejka wsyp jedną łyżeczkę sody oczyszczonej. Zapal umieszczoną we wnętrzu zlewki świeczkę. Wylot balonu nałóż na szyjkę butelki, a następnie dodaj do octu sodę. Zebranym w balonie gazem napełnij drugą zlewkę. „Przelej” dwutlenek węgla do zlewki z zapaloną świeczką.
Does the carbon dioxide obtained from baking soda and vinegar support the burning of the candle?
Select one of the hypotheses and justify it.
Carbon dioxide is a gas that supports the burning of a candle.
Carbon dioxide is a gas that does not supports the burning of a candle.
plastic bottle,
baking soda,
vinegar,
balloon,
tea light,
two beakers,
funnel.
Pour a small amount of vinegar into the bottle (maximum 1/3 of the bottle's volume).
Add a teaspoon of baking soda into the balloon using a funnel.
Light a candle placed inside the beaker.
Place the balloon outlet on the neck of the bottle, and then add soda to the vinegar.
Fill the second beaker with gas accumulated in the balloon.
„Transfer” carbon dioxide into a beaker with a lighted candle.
Observe the changes.
What do we know about carbon dioxide this far?
In what order will carbon dioxide blow out the candles placed in a beaker at different heights?
Select one of the hypotheses and justify it.
The carbon dioxide collected in the beaker will blow out the candles, starting from the lowest one. The carbon dioxide collected in the beaker will blow out the candles, starting from the highest one.
calcium carbonate,
hydrochloric acid,
conical flask,
a beaker with candles located at different heights,
cork with dropper and drain tube.
Add diluted hydrochloric acid to the flask with calcium carbonate.
Cork the flask with using cork with dropper and drain tube.
Carefully light the candles, then place the end of the drain tube in the beaker.
Observe the changes.
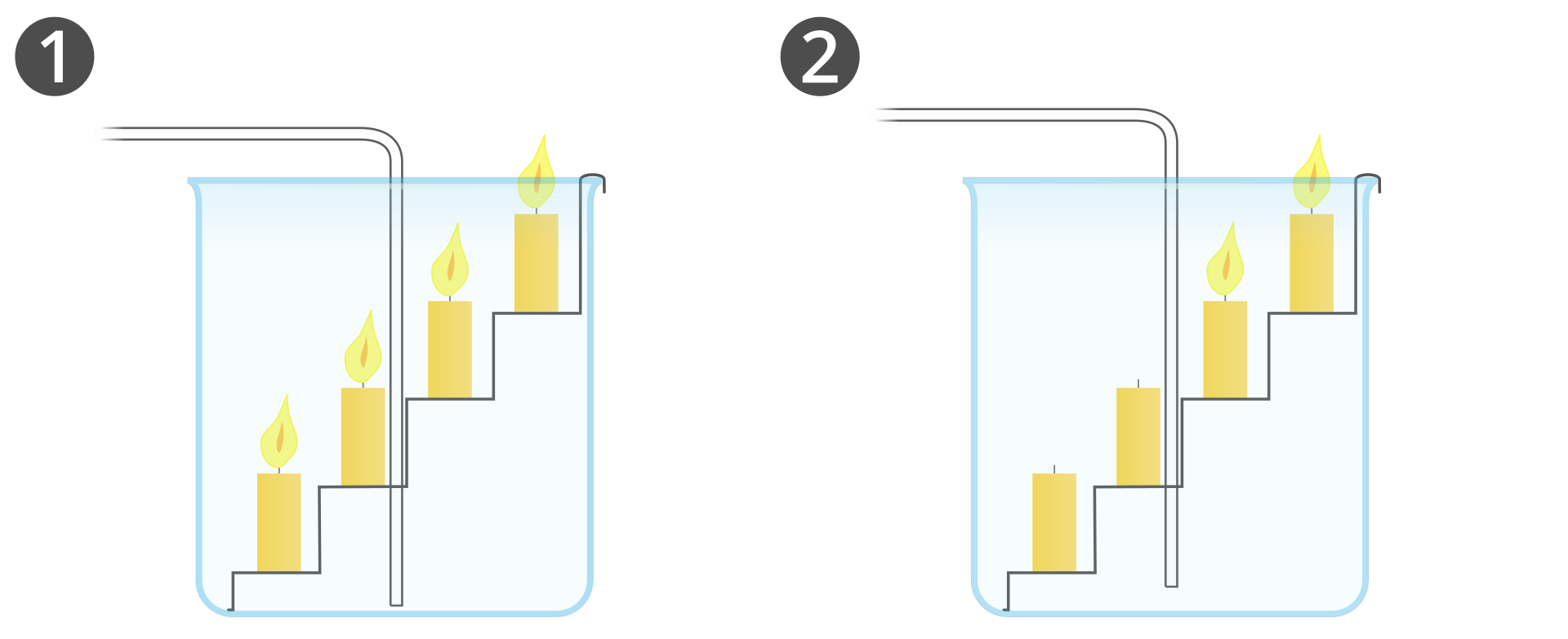
As a result of the reaction of calcium carbonate with hydrochloric acid gas bubbles are emitted. The reaction is described by the equation
The beaker gradually fills up with gas that accumulates on the bottom of the beaker, blowing out the candles. First, the lowest candle is blown out, and at the end – the highest one. The emitted gas is carbon dioxide, which does not support combustion and is heavier than air.
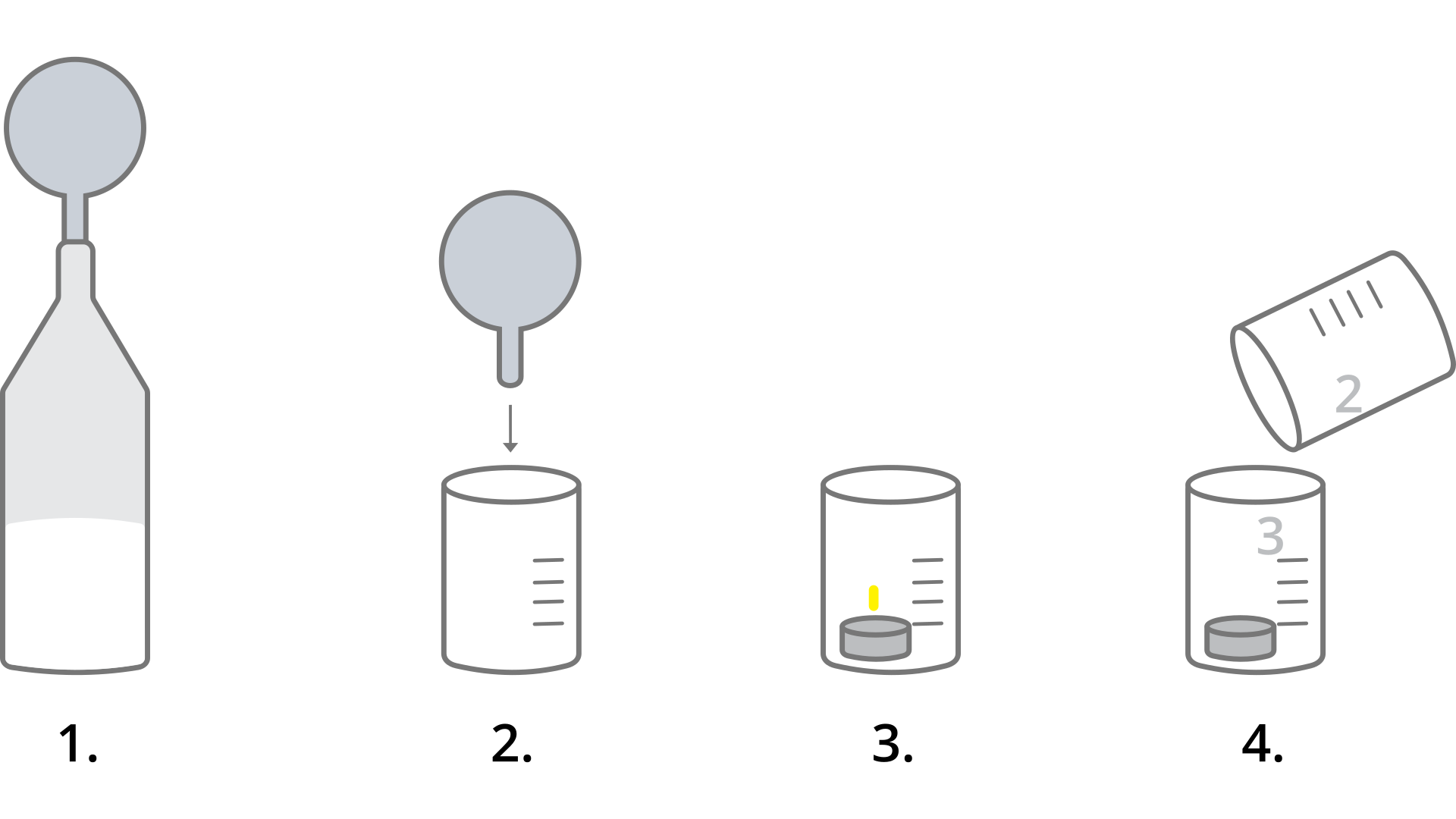
Suggest a way to detect the presence of carbon dioxide. Write down your suggestion here or in a notebook.
How the presence of carbon dioxide can be detected?
Select one of the hypotheses and justify it.
Carbon dioxide, when introduced into limewater, causes a colour reaction. Carbon dioxide, when introduced into limewater, causes its clouding.
two test tubes or small beakers,
a straw,
limewater.
Pour the same amount of limewater into both test tubes or small beakers.
Insert one end of the tube or straw into one of them and blow in the air out of the lungs.
Observe the changes.
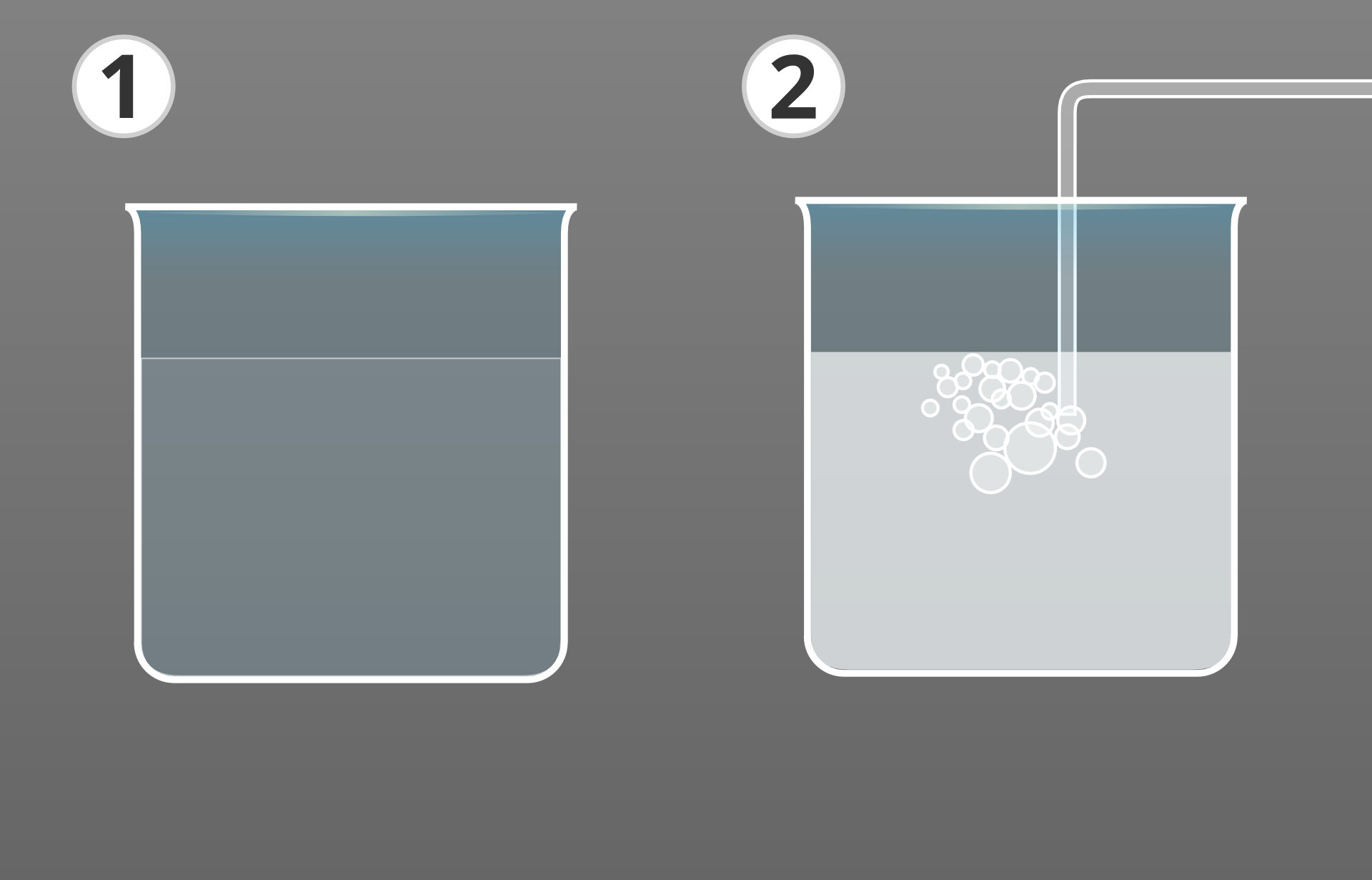
Plan an experiment, make a hypothesis and explore which gas:
is present in sparkling water,
is released as a result of dissolving the effervescent tablet in water.
Note down observations and conclusions.
Dry iceDry ice and sublimationsublimation
Solid looks like ice, but instead of melting, it sublimates, forming carbon dioxide, hence the name dry ice.

Application of carbon dioxide
Carbon dioxide is a gas that is well soluble in water, so it has been used in the food industry for the production of fizzy drinks. Carbon dioxide does not support combustion and blows out fire, so it is used in foam extinguishers (as a carrier gas) and in carbon‑dioxide extinguishers (as a fire extinguisher).
Solidified carbon dioxide is a cooling agent used during transport and storage of food products, in particular fruit and vegetables. Due to the low temperature, it contributes to the inhibition of the growth of bacteria and fungi. Dry ice is also used in medicine for storing preparations and vaccines and in cryotherapy (cold treatment).
What is the use of dishwashing soap in the experiment?
Dishwashing soap causes the formation of carbon dioxide. The addition of dishwashing soap causes the formation foam.
plastic bottle with dispenser,
baking soda,
citric acid,
dishwashing soap,
beaker,
candle.
Pour 200 ml of water into an empty bottle.
Add two teaspoons of baking soda and shake.
Add about 30 ml of dishwashing soap and shake again.
Using a funnel, quickly add three heaping teaspoons of citric acid.
Cork the bottle very quickly.
On a foil, for example a painter foil, place a burning candle in a beaker, then shake the contents of the bottle, point the outlet of the home‑made fire extinguisher over the candle and pull the cork to open it.
Sparkling water
In 1767, John Priestley discovered a way to saturate water with carbon dioxide by hanging a bowl of water over a vat of beer. The water prepared in this way had a pleasant taste, and Priestley announced his discovery in the press. In the article, he described the production of sparkling water, consisting of adding acid to chalk to produce carbon dioxide, and then saturating water with it.
At the end of the 18th century, Johann J. Schweppe mastered this technology and sparkling water was produced by saturating the water with carbon dioxide under pressure. Higher pressure increases the solubility of carbon dioxide compared to atmospheric pressure conditions. After opening the bottle, the pressure is levelled, which causes the gas to escape from the solution, creating characteristic bubbles (source).
Summary
Carbon dioxide is a component of the air. Its content in the air is variable. It is created in the processes of breathing, fermentation and combustion.
Under the influence of sunlight, in the presence of chlorophyll found in cells, carbon dioxide with the help of water transforms into oxygen and glucose. This process is called photosynthesis.
Carbon dioxide is a colourless gas, well soluble in water, with a density about 1.5 times higher than the density of air. It does not combust and does not support combustion.
Carbon dioxide is used as a refrigerant, extinguishing agent, gas for filling life jackets and salvage pontoons.
Carbon dioxide is not a toxic gas, but its high concentration can even cause death.
Human exhales about 416 kg of carbon dioxide annually from the lungs. Calculate how many kilograms of is exhaled by the human during the week.
Keywords
Carbon dioxide, sublimation, limewater, dry ice, air
Glossary
reakcja charakterystyczna – reakcja umożliwiająca wykrycie (identyfikację) danej substancji
sublimacja – przemiana fazowa polegająca na bezpośrednim przejściu substancji z fazy stałej do stanu gazowego z pominięciem fazy ciekłej
suchy lód – zestalony tlenek węgla(IV)
woda wapienna – nasycony roztwór wodorotlenku wapnia w wodzie, odczynnik laboratoryjny, który umożliwia identyfikację tlenku węgla(IV)