Principle of mass conservation
that chemical reactions involve changes resulting in new substances;
that chemical equation reflects chemical changes using symbols of elements and formulas of chemical compounds;
that substrates are substances involved in a chemical reaction and that products are substances obtained as a result of chemical reaction;
how to determine mass ratio in a chemical compound based on its formula.
to state the principle of mass conservation and interpret it;
to solve tasks using the principle of mass conservation.
Nagranie dostępne na portalu epodreczniki.pl
Nagranie dźwiękowe abstraktu. Czy masa i energia substratów zmienia się podczas reakcji chemicznej?
Does the mass and energy of substrates change during a chemical reaction?
While observing the course of chemical reactions, we can describe effects that occur during it, for example changes in colour, sounds, light emission. Sometimes one can also get impression that the quantity of substances involved in the reaction is decreasing or increasing.
Masses of substrates and products were compared already in the 18th century. Due to these studies, conducted independently by two chemists, Mikhail Lomonosov from Russia (1756) and Antoine Lavoisier from France (1785), a general law of nature was formulated. It was called principle of mass conservationprinciple of mass conservation. In line with this law, total mass of substrates is equal to total mass of products in an isolated system (in which reaction products and energy does not leave this system). This means that the same mass of substrates produces the same mass of products; that is mass of substances involved in chemical reaction does not change.
The need to balance chemical equations results in fact from the principle of mass conservation. If the total mass of substrates is to be equal to the total mass of products, numbers of atoms of the same type on both sides of the equation have to be identical.
Nowadays the law of mass conservation is extended by the energy of ingredients. Reagents are characterized by their own internal energy called the resting mass. However, due to the fact that the resting mass of the system or chemical reaction contributes not only to the rest masses of the components, but also all forms of internal energy related to the movement of elemental atoms in space and their mutual interactions, the rest mass of the system is equal to the sum of masses its components and their energy.
During chemical reactions, the structure of the resting mass of the system may change, eg by reducing the sum of the rest masses of its components, and increasing the sum of their energy.
For closed systems but not insulated, the right to maintain the rest mass is not satisfied, because there is a flow of energy between the system and the environment, which results in a change in the rest mass of the system.
However, during chemical reactions, the amounts of energy exchanged are so small that the mass change of the system is not detectable by standard methods, hence the stability of the mass of the reaction system is assumed. In chemical reaction, the sum of the masses of products and substrates are the same.
Before you watch the video “How to control mass of substances involved in the reaction of baking soda with vinegar”, write down a research question and a hypothesis.

Film dostępny na portalu epodreczniki.pl
Nagranie filmowe przedstawiające eksperyment- kontrola masy substancji podczas reakcji sody oczyszczonej z octem. Do przeprowadzenia eksperymentu potrzebne są: waga laboratoryjna, proszek do pieczenia, ocet winny, mała kolba pomiarowa, balon, łyżeczka. Przebieg doświadczenia: dodaj 2–3 łyżeczki sody oczyszczonej do balonu, następnie do kolby miarowej wlej 20–30 cm3 octu, później umieść balon na szyjce kolby, upewnij się, że soda nie dostanie się do kolby, następnie umieść zestaw na wadze laboratoryjnej, gdy masa kolby zostanie ustawiona na wadze, podnieś balon i dodaj sodę do octu. Ponownie sprawdź wskazanie wagi.
Is mass of products greater, smaller than or the same as the mass of its substrates?
Mass of products is equal to mass of the substrates used.
laboratory scale,
baking soda,
vinegar,
small measuring flask,
balloon,
teaspoon.
Add 2–3 teaspoons of baking soda to the balloon.
Pour 20–30 cmIndeks górny 33 of vinegar to the measuring flask.
Place the balloon on the flask neck. Make sure that the soda does not get inside the flask.
Put the set on the laboratory scale.
When weight of the flask is set on the balance, lift the balloon and add soda to vinegar.
Observe indications of the laboratory scale.
How can the principle of mass conservation be used in chemical calculations?
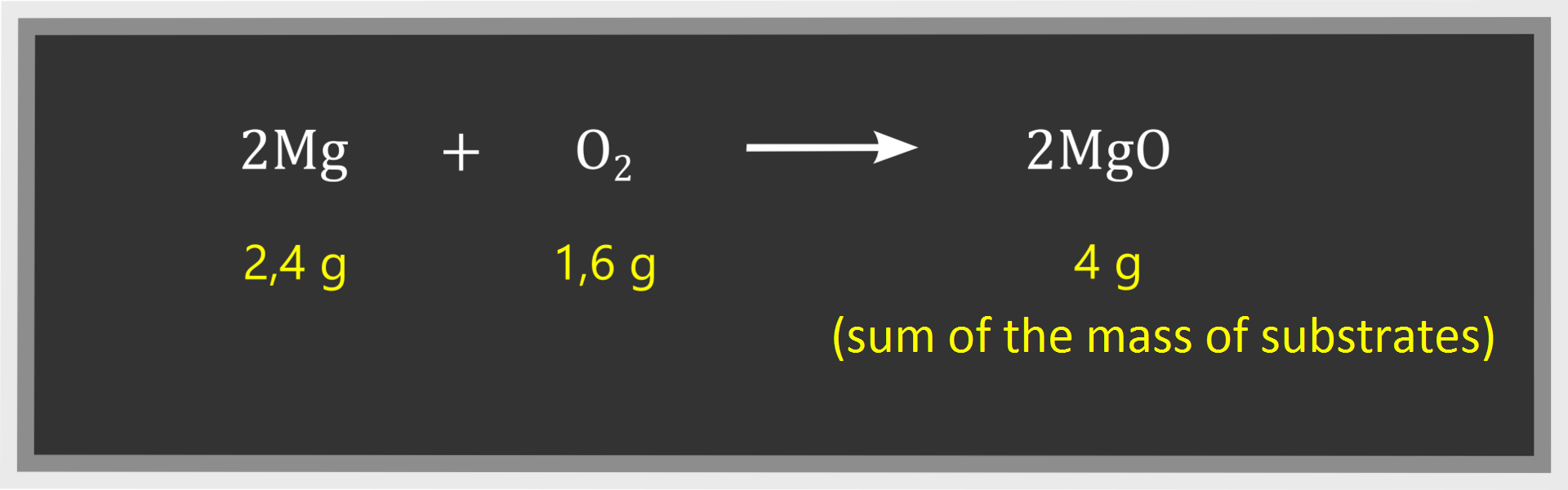
The principle of mass conservation helps determine mass of one substance if we know masses of other substrates and products. If you know this principle, you can calculate for example the quantity of products resulting from a given mass of substrates. For example, if we know that 2.4 g of magnesium and 1.6 of oxygen were involved in the reaction, we can easily determine that of magnesium oxide was produced in this chemical reaction:
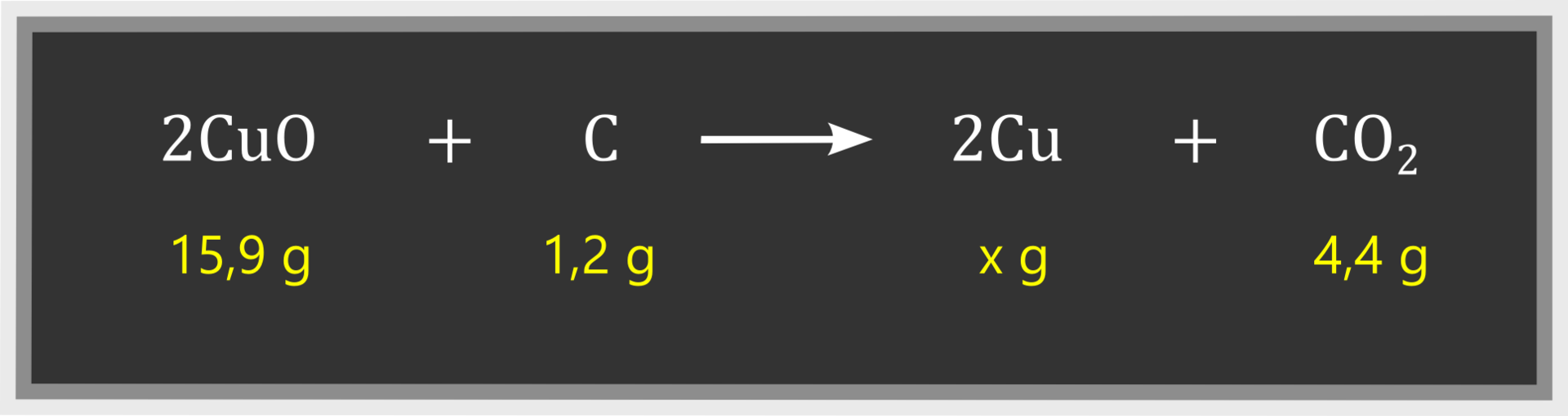
In case of another reaction – exchange reaction of copper(II) oxide with carbon – we can determine mass of copper if we know masses of substrates and mass of the second product:
In line with the principle of mass conservation, total mass of substrates is to be equal to total mass of products:
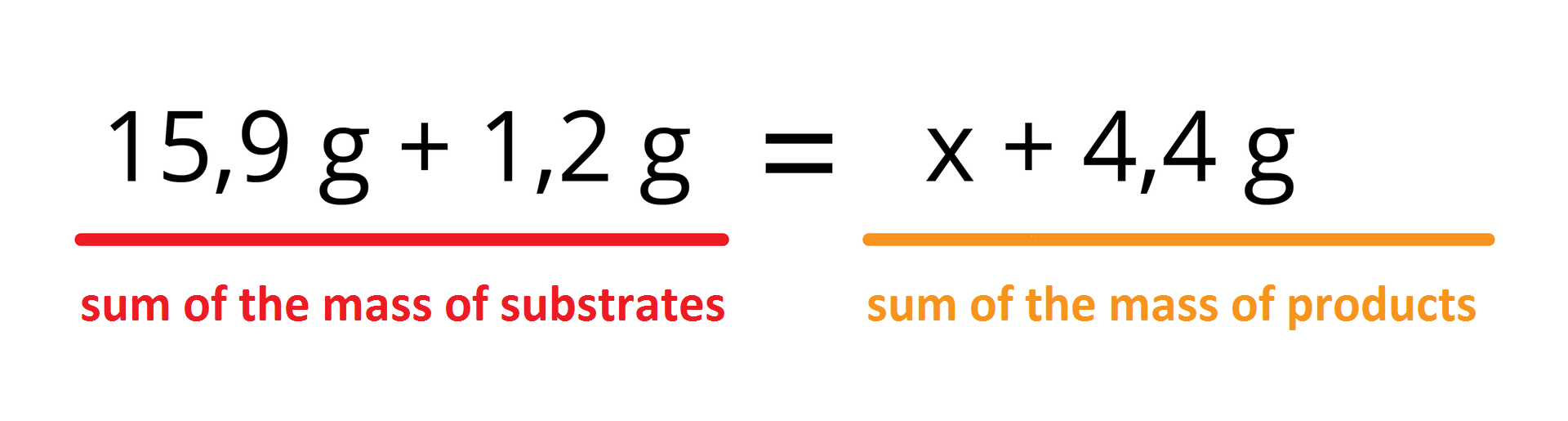
After rearranging the equation and making calculations we will know the mass of copper:
Using the principle of mass conservation, we can conclude that 12.7 g of copper will be produced in a reaction of 15.9 g of copper(II) oxide and 1.2 g of carbon.
An experiment was conducted. Hydrogen chloride was added to water and then magnesium chips were added to the mixture. It was observed that a gas was released. It was hydrogen. During the experiment 2.4 g of magnesium and 7.3 of hydrochloric acid reacted with each other. Hydrogen and magnesium chloride were products of this reaction. Mass of magnesium chloride was determined. It amounted to 9.5 g. The reaction is described with the following equation:
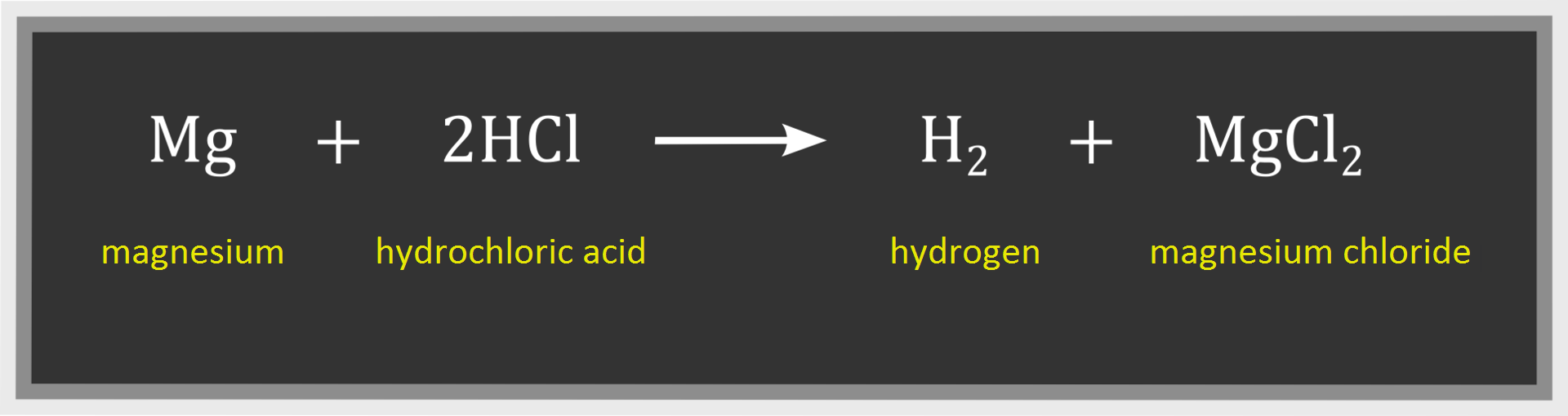
Calculate mass of hydrogen produced in this reaction and determine the number of molecules of this gas.
Mark true statements.
- 252 g of calcium oxide will be produced as a result of thermal decomposition of 450 g of calcium carbonate, during which 198 g of carbon dioxide was obtained.
- According to the principle of mass conservation, total mass of all substrates in a reaction is not equal to total mass of all resulting products.
- According to the principle of mass conservation, an isolated system in which a reaction is carried out is a system that does not exchange mass with environment, but only energy.
- 8.96 dm3 of oxygen with density of 1.43 g/dm3 and 0.8 g of hydrogen were obtained as a result of decomposition of 14.4 g of water.
Summary
According to the principle of mass conservation it is assumed that in each reaction total mass of substrates is equal to total mass of resulting products.
Mass of each substrate or products can be calculated based on the principle of mass conservation, if you know masses of the other ones.
According to the law of definite proportion, mass ratio of elements in a chemical compound is always constant and does not depend on its source and method of preparation (each chemical compound always contains its component elements in fixed qualitative and quantitative ratio).
If you know mass ratio of chemical elements in a compound, you can calculate mass of chemical elements in given amount of this compound.
Molecular formula of given compound may be determined based on mass ratio of its component elements.
Keywords
Principle of mass conservation, reaction, substance
Glossary
prawo odnoszące się do stosunków masowych w związkach chemicznych, zgodnie z którym stosunek masowy pierwiastków w związku chemicznym jest zawsze stały i niezależny od sposobu oraz miejsca jego otrzymania. Prawo to nie jest spełnione w przypadku bertolidów (związków niestechiometrycznych). Przyczyną zmiennej zawartości różnych pierwiastków w związku mogą być defekty sieci krystalicznej lub występowanie nadmiaru atomów jednego ze składników, nietworzących wiązań chemicznych.
prawo zachowania masy – reguła, która mówi, że w układzie zamkniętym w przypadku każdej reakcji chemicznej całkowita masa substratów jest równa łącznej masie produktów.