Ubiquitous silica
that silicon dioxide has a molecular formula ;
acid, alkaline and neutral oxides are distinguished; that the location of the chemical element in the periodic table depends on the structure of its atom;
that most of the chemical elements found in nature are mixtures of isotopes.
to study and describe the physical and chemical properties of silicon dioxide;
to exchange the occurring varietes in nature and determine their application;
to explain why quartz glass is used for the production of laboratory crucibles and lamps used in medicine for the treatment of, among others, skin diseases (mainly psoriasis and acne);
to justify the method of storage of hydrofluoric acid.
The occurrence of silicon dioxide in nature
The outermost and best‑known layer of the Earth's rock is the Earth's crust. Only 11 elements occur in it in an amount greater than 0.1%, which is as much as 99,895% of the mass of this part of the globe. Watch the video from the e‑textbook and find out what elements the earth's crust consists of.
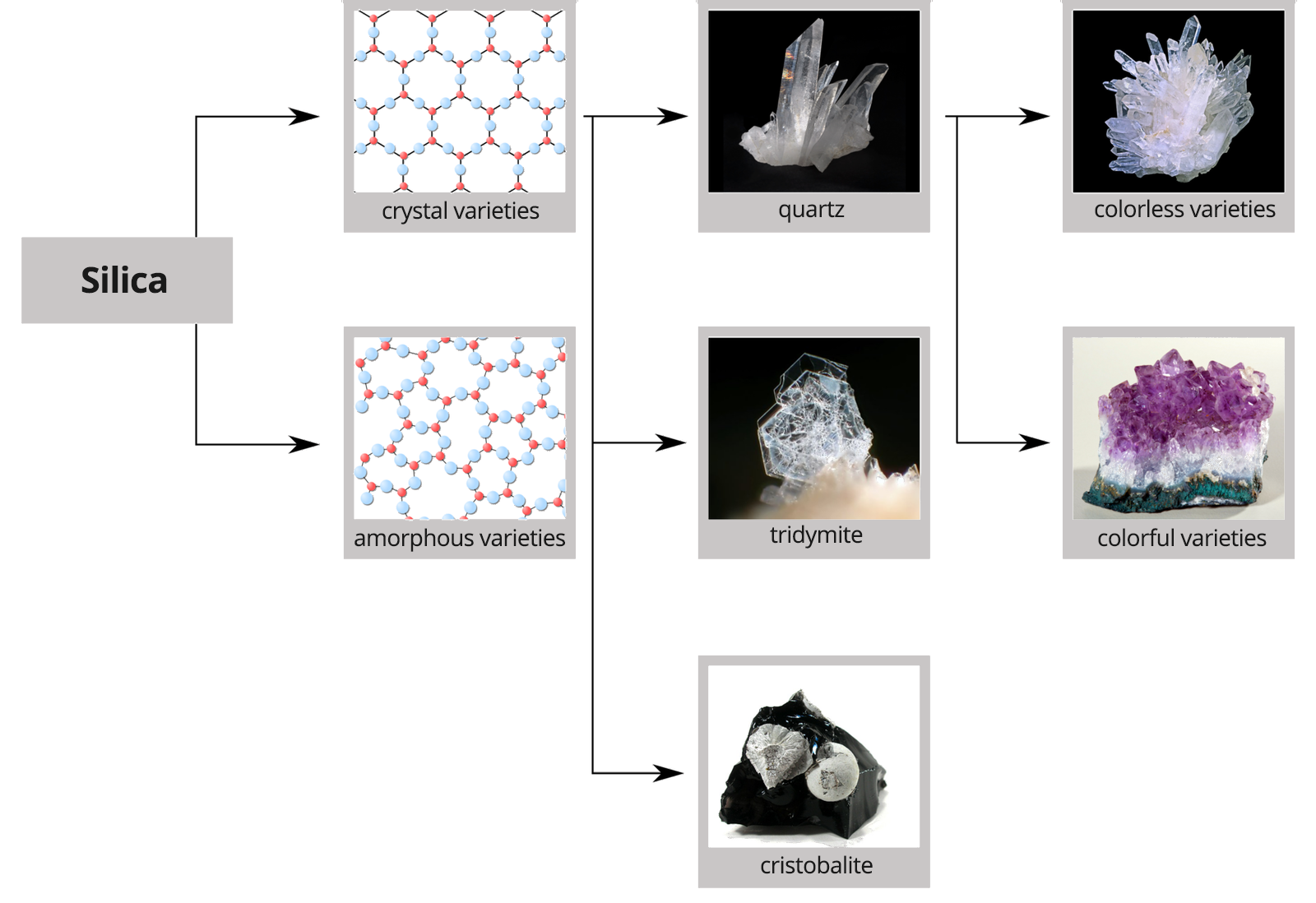
The second most‑spread element in the earth's crust is silicon, and silicon dioxide , commonly known as silica, is as common as water. Silica is the main component of sand, rocks and soils. In nature, we encounter it both in a crystalline form, with an ordered internal structure (as quartz, tridymite and cristobalite), as well as in the non‑crystalline form, i.e. amorphous (in the case of e.g. agates, opals, jasper, onyx, diatomaceous earth). These varietes are characterized by the lack of an ordered internal structure.
Physical properties of silicon dioxide
Before you conduct or watch the teacher conducting the experiment „Study of physical properties of silica”, write down the research question and the hypothesis. Make a note of your observations and concluding remarks from the study.
What physical properties has the basic component of sand?
Silicon dioxide is a hard, insoluble solid that does not conduct electricity.
silicon dioxide in the form of quartz sand,
water,
beakers,
glass rod,
a piece of glass,
2 graphite electrodes,
wire,
battery,
light bulb.
Split small amount of silicon dioxide into 3 portions.
Place the first one in a beaker, pour water and mix the contents with a baguette.
Use the second part of the silica to check if the substance is scratching glass. To do this, move the sand with your fingers over a piece of glass.
Put the third portion into a beaker, in which two electrodes should be placed, connected by a wire with a bulb and with a battery, closing the electric circuit.
Chemical properties of silicon dioxide
Write the research question and hypothesis before watching the teacher conducting ‘Study of silica chemical properties’ experiment. While watching pay an attention to what is going on with compounds used in the experiment. Write down your observations and concluding remarks.

Film dostępny na portalu epodreczniki.pl
Film przedstawia eksperyment sprawdzający chemiczne właściwości tlenku krzemu cztery. Aby zbadać z jakimi substancjami reaguje krzemionka, potrzebujemy: czystego piasku kwarcowego, sprzętu laboratoryjnego: trzech probówek, trzech korków, palnika, trzech wkraplaczy i łyżki oraz odczynników: wody destylowanej, stężonego roztworu wodorotlenku sodu, stężonego roztworu kwasu chlorowodorowego. Do trzech probówek dodać piasek kwarcowy. Następnie dodać wodę destylowaną do pierwszej probówki, roztwór wodorotlenku sodu do drugiej probówki i roztwór kwasu chlorowodorowego do trzeciej probówki. Zamknąć probówki szczelnie za pomocą korków i wymieszać zawartość. Następnie podgrzać zawartość probówek w płomieniu palnika. Należy pamiętać o trzymaniu probówek w łapie i wstrząsaniu zawartości oraz o otwarciu probówek podczas ogrzewania.
What chemical properties does the basic component of sand have?
Silicon dioxide is a substance with low chemical activity and acidic properties.
silicon dioxide in the form of quartz sand,
water,
concentrated solution of sodium hydroxide,
concentrated hydrochloric acid,
test tubes,
plugs,
test tube holder,
gas burner,
dropping funnel,
spoon.
Before you start this experiment, wear safety goggles and gloves.
Insert quartz sand into three test tubes.
Add water to the first tube, enter a concentrated solution of sodium hydroxide to the second one, and concentrated hydrochloric acid to the third.
Plug the test tubes and mix their contents.
Unplug the tubes and heat them in the flame of the burner.
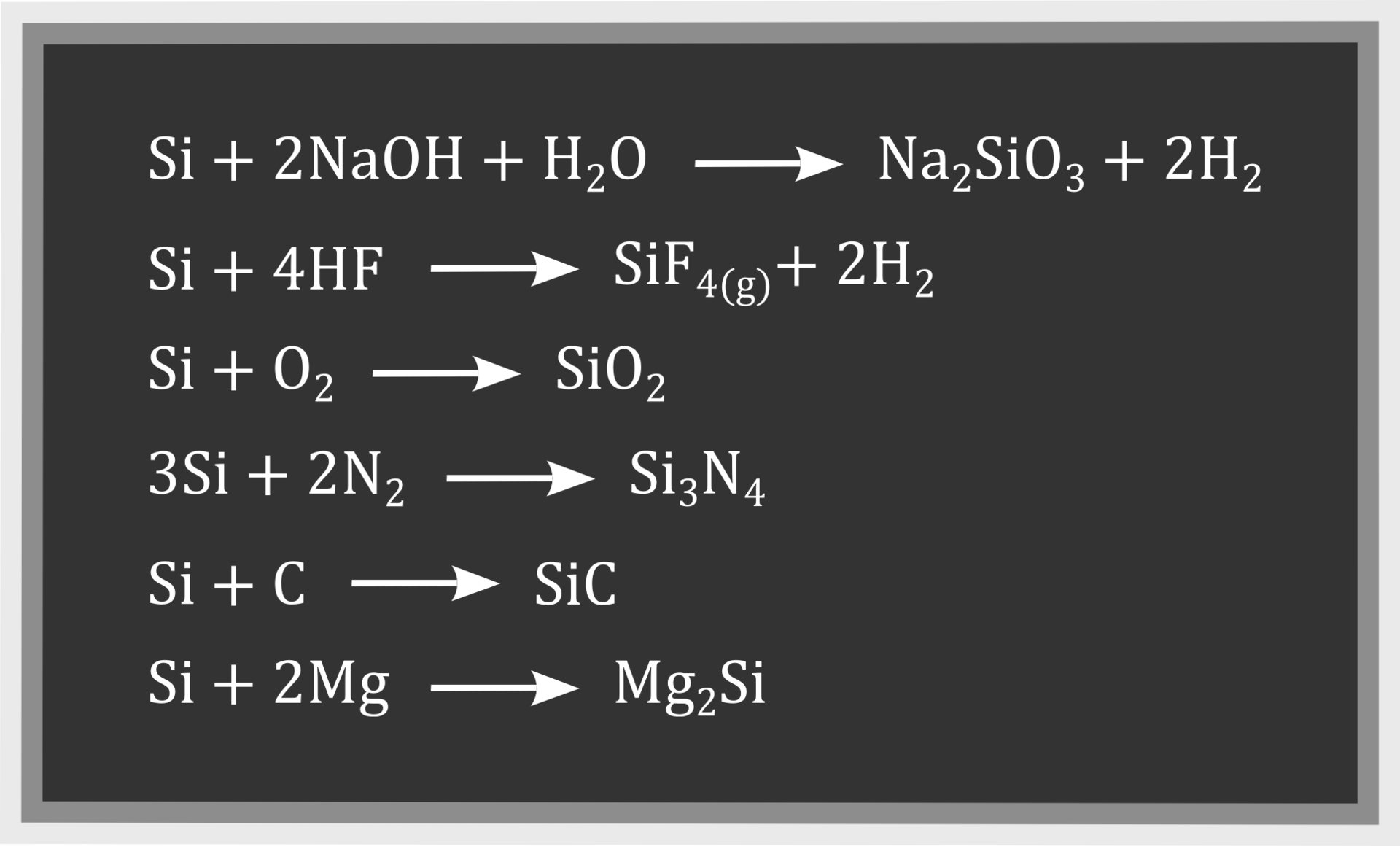
Silicon dioxide does not react with water or hydrochloric acid, even after heating. It digests under the influence of sodium hydroxide, which can be described by the reaction equation:
Silicon dioxide is an acid oxide with low chemical activity. In addition (which we have not had the opportunity to observe) analogous conversions occur during the melting of silicon dioxide with hydroxides, basic oxides and salts, e.g.
Does not react with acids, except for hydrofluoric acid:
Does not decompose under the influence of high temperature, but can then react with some elements, e.g.:
carbon
magnesium
These chemical reactions are used to obtain silicon.
Application of silicon dioxide
From the earliest times people have used the unusual properties of silica. A mineral called flint, thanks to its hardness, was used to strike a fire. It is brittle, and its shards have sharp edges, which is why it was used by the Paleolithic people as a raw material for the production of stone tools. Today flint is a raw material used in the ceramic industry and used for the production of paints. Coatings with silicate paints are durable, resistant to moisture and have high mechanical resistance. They are completely non‑flammable and resistant to the development of microorganisms. From striped flint due to the rarity of its occurrence, aesthetic values and appropriate hardness, original jewelry and decorations are created.
Melted quartz upon slow cooling creates so‑called quartz glass, which is used for production of, among others, lamps in solariums, hospital rooms, fiber optics, laboratory crucibles. Quartz crystals have piezoelectric properties. When we place them in a variable electric field, these crystals shrink and expand, emitting ultrasounds. Ultrasounds allow to obtain images of various objects, which was used in sonars – to locate icebergs, schools of fish, as well as in medicine - for USG examination. Quartz crystals are also used for the production of extremely accurate clocks and lighters and igniters – due to the piezoelectric effect, which consists in the formation of electric charges on the surface of this crystal, due to mechanical stresses. As a decorative stone it is used in jewelry. In the optical industry, it is used for the production of lenses and prisms.
Sand is the basic component of mortar and cement and a raw material for the production of ceramic products, as well as various types of glass, e.g. window, decorative, water. In addition, silicon and its alloys are obtained from sand, it produces carborundum (SiC – hard grinding material).
Silicon - the element of life and electronics
Physical and chemical properties of silicon
Silicon is a dark gray solid with a metallic gloss. It creates crystals similar to diamond crystals. It is a semiconductor. It is a hard element (6.5 on the Mohs scale) and fragile. It has a high melting point (1863 K), during which it reduces its volume, similar to water. It is not chemically active. Does not react with water nor acids, except for hydrofluoric acid. At room temperature, silicon reacts only with fluorine to form silicon tetrafluoride – . After heating, it reacts with other halogens, with oxygen and nitrogen, so that at temperatures of about 1000 K oxide is formed and nitride, and at temperatures above 2300 K it reacts, among others with carbon, forming a carbide. At high temperatures, it also reacts with metals, forming silicates, e.g. . In solutions of bases, silicon is dissolved with the evolution of hydrogen.
The correct formula of quartz:
- SiO2
- Na2SiO3
- Mg2Si
- Si2O
Silica reacts with:
- H2O
- HF
- HCl
- H2SO4
Varieties of quartz are:
- calcite, agate, tiger eye, rock crystal
- amethyst, agate, tiger eye, anhydrite
- amethyst, agate, tiger eye, rock crystal
- amethyst, agate, corundum, rock crystal
Summary
Silicon dioxide occurs in nature in crystalline form, mainly as quartz, and amorphous as opal and diatomaceous soil.
Silicon dioxide is a solid, crystalline, colorless, insoluble in water and other common solvents. It is a substance with a high melting point and high hardness.
Silicon dioxide has low chemical activity; it does not decompose under the influence of high temperature, it does not react with water, acids (except for hydrofluoric acid), after heating it reacts with oxides and hydroxides of metals and with few elements, e.g. with carbon, magnesium. It is classified as an acidic oxide.
Keywords
silica, silicon, sand, Mohs hardness scale, piezoelectricity
Glossary
tlenek krzemu(IV)
minerały – naturalne, jednorodne składniki skorupy ziemskiej o charakterystycznym składzie i specyficznych właściwościach fizycznych; większość z nich jest częścią ciał krystalicznych o uporządkowanej budowie wewnętrznej, w której atomy i jony zajmują ściśle określone miejsce, tworząc sieć przestrzenną; minerały łączą się ze sobą w formy zwane skałami
skala twardości Mohsa – skala twardości minerałów opracowana przez niemieckiego fizyka i chemika − Friedricha Mohsa w roku 1812; dziesięciostopniowa skala stosowana do określania stopnia odporności twardszych minerałów na zarysowania przez materiały bardziej miękkie; pozwala określić, który minerał od innego, ale nie określa, o ile jest twardszy
skała – naturalny zespół jednego lub wielu różnych minerałów powstały w wyniku różnych procesów geologicznych lub kosmologicznych, tworzący podstawowy składnik skorupy ziemskiej