Water and its properties – molecular structure
that water at room temperature is a colorless and odorless liquid;
that water is a chemical compound made up of particles;
that polar covalent bonds are present between the hydrogen atoms and the oxygen atom in water molecule;
that water circulating in nature forms solutions of various chemical composition.
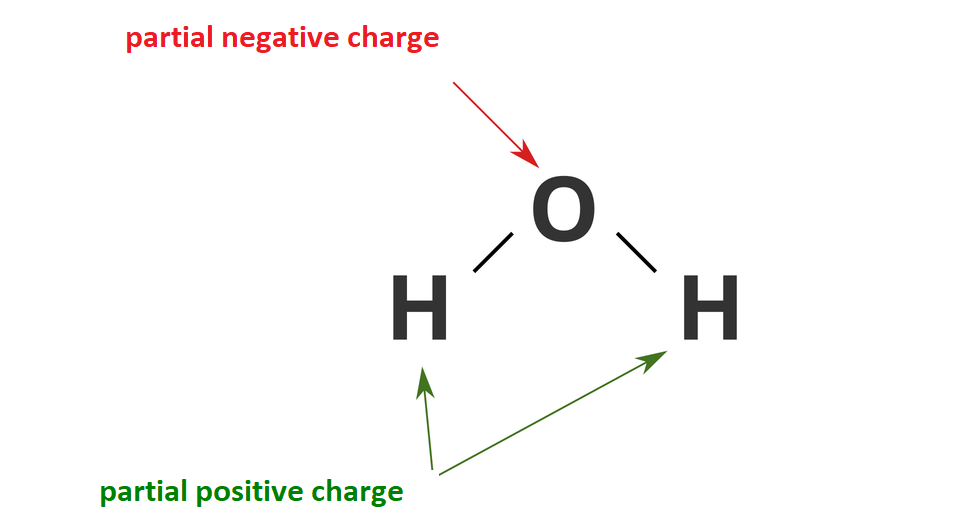
to explain the term “polarity of the water molecule”;
to present the interactions between water molecules;
to carry out an experiment, the purpose of which is to investigate whether a given substance is soluble in water or not.
The structure of the water molecule
Water is a substance made of molecules. Each molecule consists of two hydrogen atoms connected with one oxygen atom. Oxygen and hydrogen atoms are connected by polar covalent bonds. Hydrogen and oxygen atoms do not lie along one line, the bonds between them form an angle of about 104.5 °.
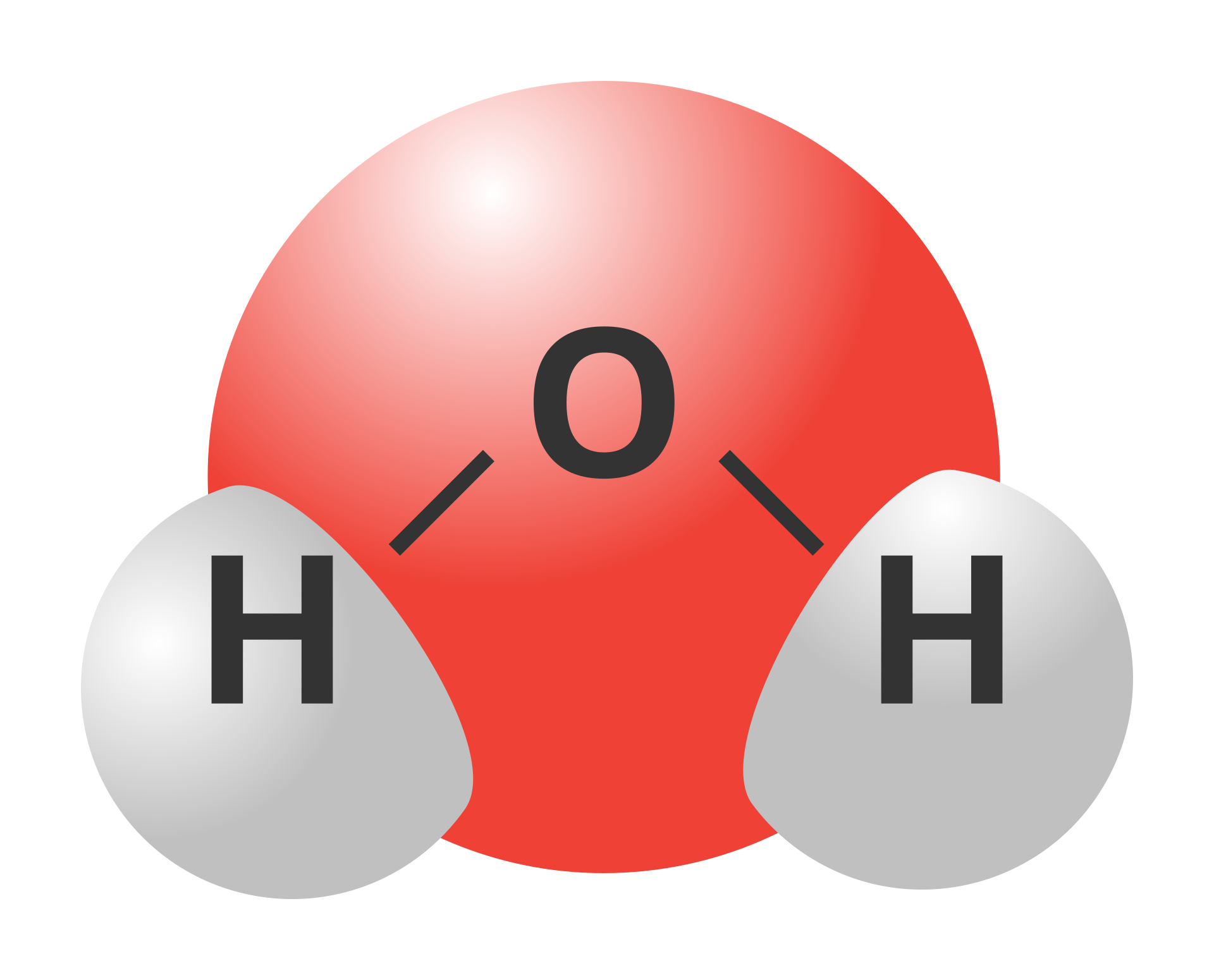
Why is the water molecule polar? Write your suggested explanation here or in your notebook.
The behaviour of water molecules in various states of matter
As we know, opposite (positive and negative) charges attract each other. This is also the case with water molecules – the hydrogen atom of one molecule can interact electrostatically with an oxygen atom of the other molecule. This phenomenon can be clearly observed in water in liquid and solid state. In water in the liquid phase, apart from free molecules, there are also clusters of molecules that form exactly in the result of electrostatic attraction. These clusters are not permanent. Some molecules are released from them, while others join the system. In the solid state, water molecules, due to electrostatic interactions, form relatively stable structures.
The phenomenon of particles (molecules, ions, atoms) interconnecting to form larger systems composed of two or more particles as a result of electrostatic interactions is called associationassociation.
How does the polar structure of water molecules affect the density of this substance?
Read the information in the table below and indicate the discrepancy between the description typical for many substances and the model of packing the molecules for water.
Expand the subsequent tabs and read the information about the states of matter.
Particle arrangement in the solid state
In the solid state, particles of the substance are located close to each other and do not move.
Particle arrangement in the liquid state
In the liquid state, the particles are located at greater distances from one another, they move and collide with one another very often.
Particle arrangement in the gaseous state
When the substance is in the gaseous state, its particles are located far away from one another, they travel along straight tracks and because of the available free space they collide less frequently than in the liquid state.
The model of packing of molecules in water
The model of packing of particles of a substance in the a) solid, b) liquid, c) gaseous state

The polar structure of water molecules has its consequences – the physical properties of water. Usually, the particles in solids are closer to each other than in liquids, and a substance in the solid state has a higher density than in the liquid state. In the case of water, solid phase molecules form structures that leave a lot of free space; this is why the distances between particles are larger in ice than in water. For this reason ice has a lower density than water in the liquid state.
State | Density |
water in the solid state at 0°C | |
water in the liquid state at approx. 4°C |
Look at the interactive diagram. What is the importance of the ice density, which is low comparing to the density of water in the liquid state, for the aquatic life on Earth?
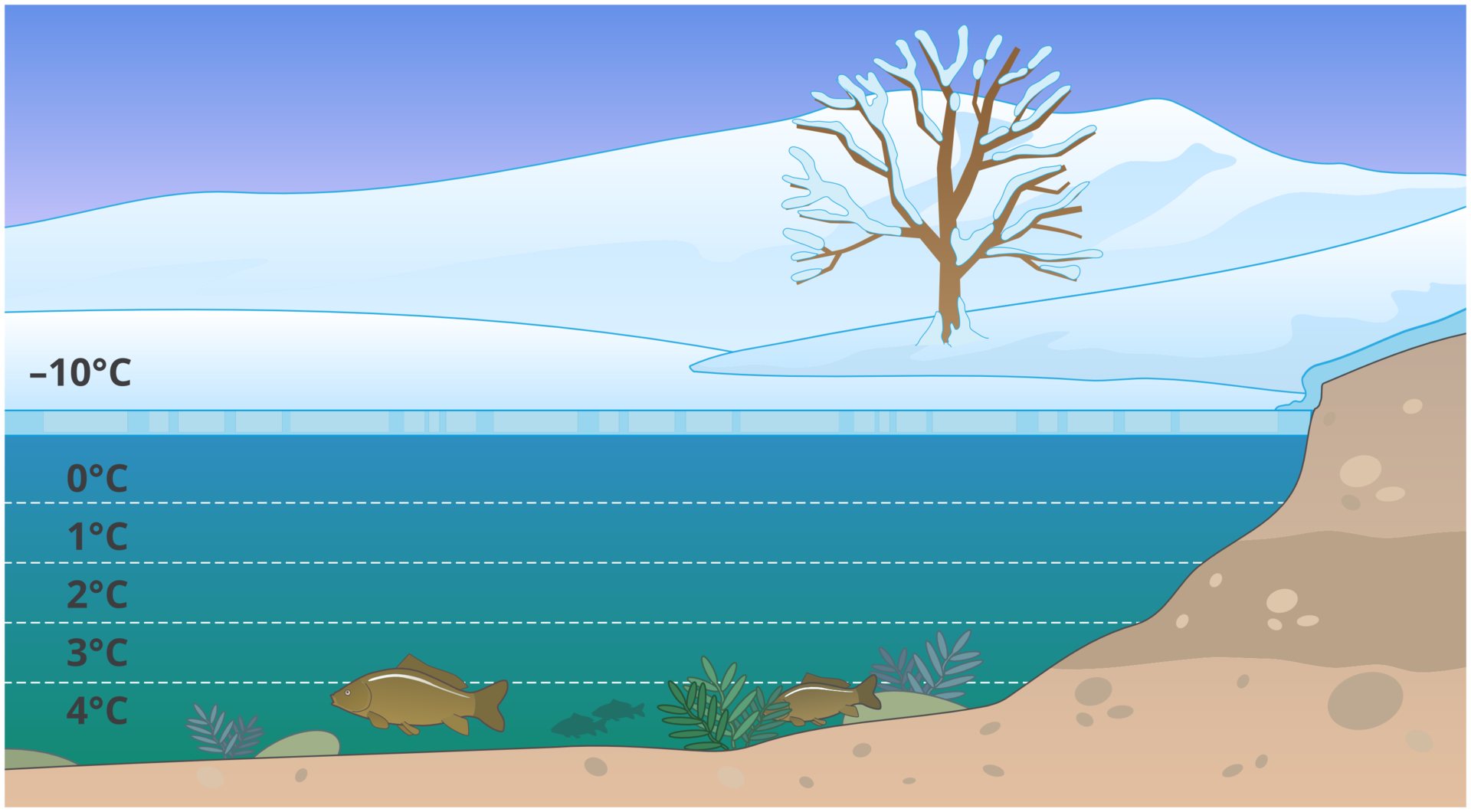
1. Ice sheet Ice that forms on the water surface insulates deeper water layers and protects them against freezing
2. Ice - why doesn’t it sink? Because water reaches the highest density at 4ºC, its further cooling or heating decreases its density. As a result, ice is light and floats on water, while normally other solids always sink in the liquids forming when they melt.
3. Water density and temperature Water reaches the highest density at 4ºC - it sinks to the bottom and as a result deep water tanks have a temperature close to 4°C. In winter, water at the bottom of the tank always has a temperature of 4°C, allowing the fish to survive this period.
4. Snow protection Snow deposited on ice may create additional thermal insulation for a lake or pond during winter. This protects the aquatic flora and fauna against excessively low temperatures
How does the polar structure of water molecules affect the melting and boiling point of this substance?
Understanding the physical properties of water will be easier if we recall a few facts about the states of matter.
When a substance changes its state from solid to liquid and then gaseous, energy must be supplied so that molecules can move away from each other. The more energy has to be supplied, the higher the melting point and the boiling point of the substance.
As ice passes into liquid, water molecules must free themselves from electrostatic interactions and then obtain energy that will allow them to move. Similarly, during vaporization, water molecules must obtain the energy needed to overcome electrostatic forces and necessary to move away from each other over long distances and to move around. Therefore, water has relatively high melting and boiling point. Much higher than substances with a similar structure, with molecules that do not interact with one another, e.g. hydrogen chloride () or hydrogen sulfide ().
Substance name | Molecular formula | Melting point | Boiling point |
water | 0°C | 100°C | |
hydrogen chloride | -114°C | -85°C | |
hydrogen sulfide | -82°C | -60°C |
Solve the crossword and explain the meaning of the result you receive.
- The boiling point of the water depends on it
- The transition of water from the gaseous state into the liquid state
- The transition of water from the solid state to the gaseous state
- E.g. the state of matter, color, density, melting point, boiling point are ... properties
- It covers about ¾ of the surface of the globe
- The transition of water from the gaseous state into the solid state
- The boiling is ... that occurs throughout the water volume
- The element whose atoms are of the smallest atomic mass, which is part of the structure of the water molecule
| 1 | |||||||||||||||||||
| 2 | |||||||||||||||||||
| 3 | |||||||||||||||||||
| 4 | |||||||||||||||||||
| 5 | |||||||||||||||||||
| 6 | |||||||||||||||||||
| 7 | |||||||||||||||||||
| 8 |
Summary
Water is a polar substance. There is a partial negative charge on the oxygen atom, while on hydrogen atoms – positive one.
Water molecules in water in the liquid phase interact with one another: the hydrogen atom of one molecule interacts electrostatically with the oxygen atom of the other molecule, and next to the free molecules there are clusters of molecules that persist due to electrostatic attraction.
Water molecules in ice form ordered structures in which they are at greater distances from one another than in water in the liquid state. Ice has lower density than water.
Water dissolves substances that are also polar. It also dissolves most of the ionic compounds.
Compare the boiling and melting points of water and, for example, hydrogen sulfide. Think about it and answer whether hydrogen sulfide molecules interact with one another electrostatically?
Keywords
association, dipole, states of matter, polar substance
Match the pairs: English words with Polish definition.
składnik roztworu; najczęściej jest to substancja występująca w przewadze, zjawisko łączenia się drobin (cząsteczek, jonów, atomów) w wyniku elektrostatycznych oddziaływań w większe układy złożone z dwóch lub większej liczby cząstek, składnik roztworu, substancja rozpuszczona w rozpuszczalniku, układ dwóch różnoimiennych ładunków elektrycznych (dodatniego i ujemnego); cząsteczka o nierównomiernym rozłożeniu cząstkowych ładunków dodatnich i ujemnych powstałych na skutek przesunięcia wspólnych par elektronowych pomiędzy atomami tworzącymi cząsteczkę, substancja zbudowana z cząsteczek, które są dipolami
| association | |
| dipole | |
| solvent | |
| polar substance | |
| solute |
Glossary
asocjacja – zjawisko łączenia się drobin (cząsteczek, jonów, atomów) w wyniku elektrostatycznych oddziaływań w większe układy złożone z dwóch lub większej liczby cząstek
dipol – układ dwóch różnoimiennych ładunków elektrycznych (dodatniego i ujemnego); cząsteczka o nierównomiernym rozłożeniu cząstkowych ładunków dodatnich i ujemnych powstałych na skutek przesunięcia wspólnych par elektronowych pomiędzy atomami tworzącymi cząsteczkę
rozpuszczalnik – składnik roztworu; najczęściej jest to substancja występująca w przewadze
substancja polarna – substancja zbudowana z cząsteczek, które są dipolami
substancja rozpuszczona – składnik roztworu, substancja rozpuszczona w rozpuszczalniku