Exergonic and endoenergetic reactions - types of reactions
that the substances involved in the chemical reaction are substrates and the substances obtained as a result thereof are products;
the course of chemical reactions is recorded using equations, symbols of elements and formulas of chemical compounds;
digits placed in front of symbols of elements and formulas of chemical compounds in the equations of chemical reaction are stoichiometric coefficients;
chemical reactions can be divided into synthesis, analysis and exchange reactions.
explain what are exergonic and endoenergetic reactions;
list examples of exergonic and endoenergetic reactions taking place in the environment;
describe chemical experiments, including: glassware and laboratory equipment; chemical reagents, apparatus schema, observations and conclusions;
apply safety rules when conducting chemical experiments.
Before you watch the movie „Reaction of aluminium with oxygen”, formulate a research question and a hypothesis.

Film dostępny na portalu epodreczniki.pl
Film przedstawia eksperyment: reakcja aluminium z tlenem. Do przeprowadzenia eksperymentu potrzebujemy: palnika Bunsena, płonącą łyżkę, kolbę Erlenmeyera, aluminium, szkiełko zegarowe. Szkiełko zegarowe zostaje podpalone na płonącej łyżce. Następnie łyżka z zawartością trafia do kolby. Wskutek tego pojawia się światło.
Is the reaction of aluminium with oxygen an exergonic or endoenergetic process?
Reaction of aluminium with oxygen is an endoenergetic reaction.
aluminium in the form of powder,
a flask filled with oxygen,
a laboratory spoon placed in a cork fitted to the neck of a conical flask,
gas burner.
Fill the laboratory spoon with powdered aluminium.
Place a spoon with aluminium in the flame of the burner.
When the aluminium powder begins to incandesce, immediately transfer it on a spoon into an oxygen flask.
Observe the changes taking place. Conduct the experiment with safety glasses on.
What types of chemical reactions are classified due to the energy effect?
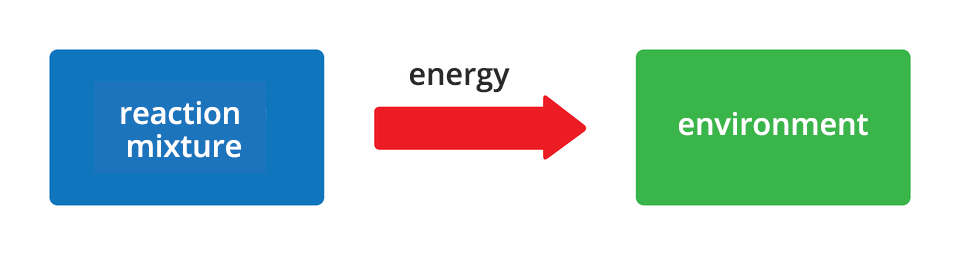
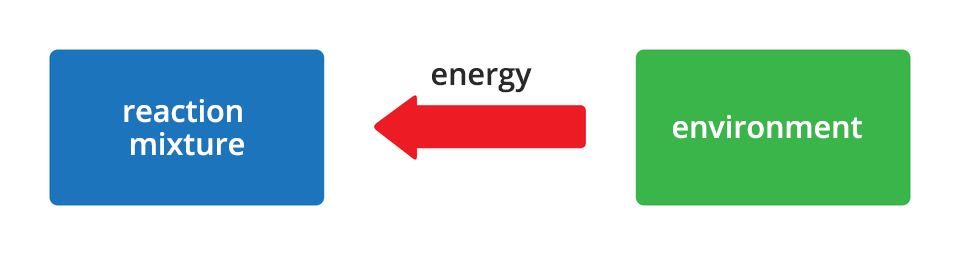
All chemical reactions are accompanied by energy effects. Sometimes these are effects visible to the naked eye, and sometimes - changes so small that these can go unnoticed. Depending on the direction of energy flow, chemical changes are divided into two groups. The reactions during which energy is released into the environment are called exergonic reactionsexergonic reactions. On the other hand, reactions accompanied by the absorption of energy from the environment are called endoenergetic reactionsendoenergetic reactions. Energy released or absorbed by reacting substances can be in the form of heat, light or work.
What are known examples of exergonic and endoenergetic reactions?
Before you watch the „Decomposition of substance contained in baking powder” demonstration, formulate a research question and a hypothesis.
Is the decomposition of the substance contained in the baking powder an exergonic or endoenergetic reaction?
Decomposition of substance contained in baking powder is endoenergetic reaction.
baking powder,
water,
test tube,
tripod,
test tube clamp
gas burner,
cork with drain hose,
teaspoon,
beaker.
Pour 2 teaspoons (3 – 4 g) of baking powder into the test tube.
Close the tube with a cork. Immerse the end of the tube in a beaker with water.
Heat the contents of the tube in the flame of the burner.
Observe the changes taking place.
After some time, gas is released from the test tube. During heating, the sodium bicarbonate contained in baking powder decomposes, which can be presented in the form of the reaction equation:
Exergonic reactions are not only those that are accompanied by heat and light emission. That are also the reactions during which work is carried out by the emitted gas. When liquids and solids react with each other to form a gas, there may be a thousand‑fold or even a larger increase in the volume of products formed in relation to the volume of substrates. The resulting gas displaces the atmospheric air. If the volume increase occurs in a very short time, an explosion occurs. Air bags in cars uses sodium azide – a substance with the formula . This chemical compound during the collision, under the influence of an electrical impulse, immediately decomposes. Nitrogen is then formed, which in a fraction of a second fills up the entire airbag and protects the passenger against additional injuries. The reaction of sodium azide analysis occurs according to the equation:

Group the named phenomena in terms of energy
Magnesium combustion, baking cake, thermal decomposition of calcium carbonate, Carbon combustion, photosynthesis process, wood burning, decomposition of mercury(II) oxide, cooking potatoes, conversions of food in the human body, mixing of sodium hydroxide with water
| Exergonic reactions | |
|---|---|
| Endoenergetic reactions |
Summary
All chemical reactions, in order to start, require a certain amount of energy. Every chemical change needs a different amount of this energy.
Due to energy effects, we divide chemical reactions into exergonic and endoenergetic.
Exergonic reactions are chemical changes during which energy from the reaction system is released into the environment in the form of heat, light or work.
Endoenergetic reactions are chemical changes that take place with energy intake from the environment.
Combustion reactions always occur with the separation of energy, which is why these are included in exergonic reactions.
Keywords
Exergonic reaction, endoenergetic reaction
Glossary
reakcja egzoenergetyczna – przemiana chemiczna, podczas której następuje wydzielenie energii z układu do otoczenia
reakcja endoenergetyczna – przemiana chemiczna, która przebiega z pobieraniem energii z otoczenia