Ammonia
what the structure and properties of the groups of selected organic compounds, such as alcohols, carboxylic acids and esters are.
what ammonia is;
what the physical and chemical properties of ammonia are;
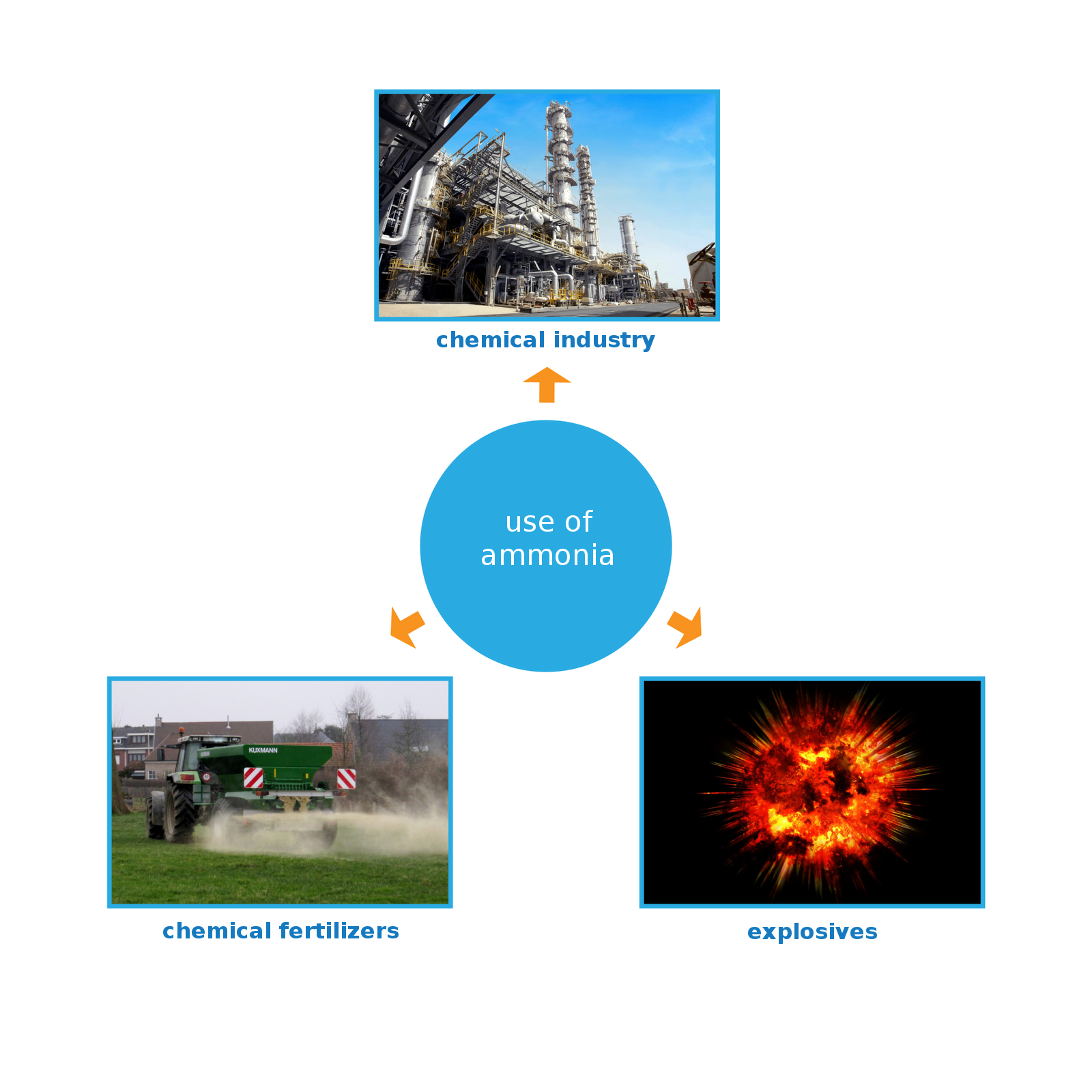
where this compound is used;
what the effect of this compound on the human body is and why we should exercise caution in contact with it.
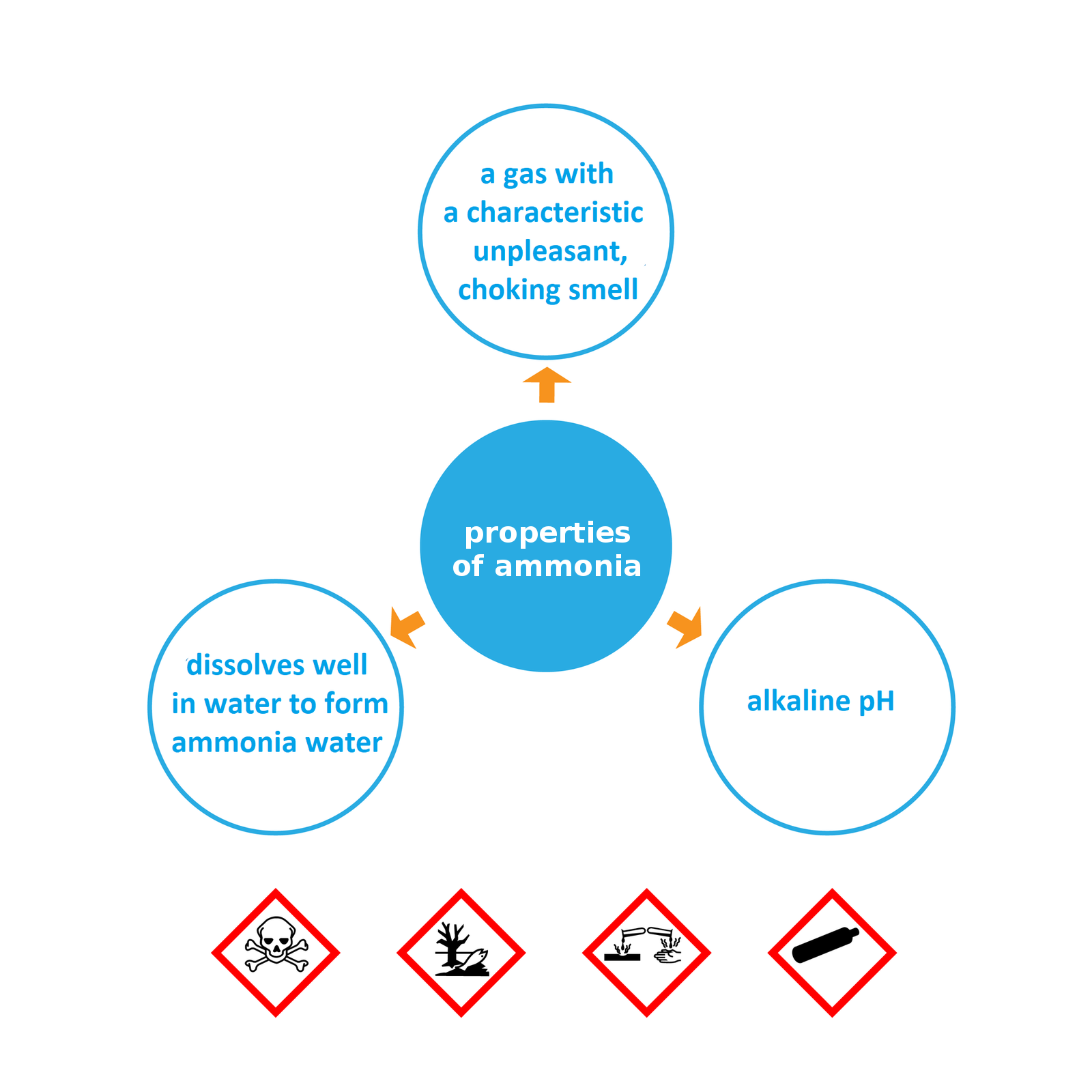
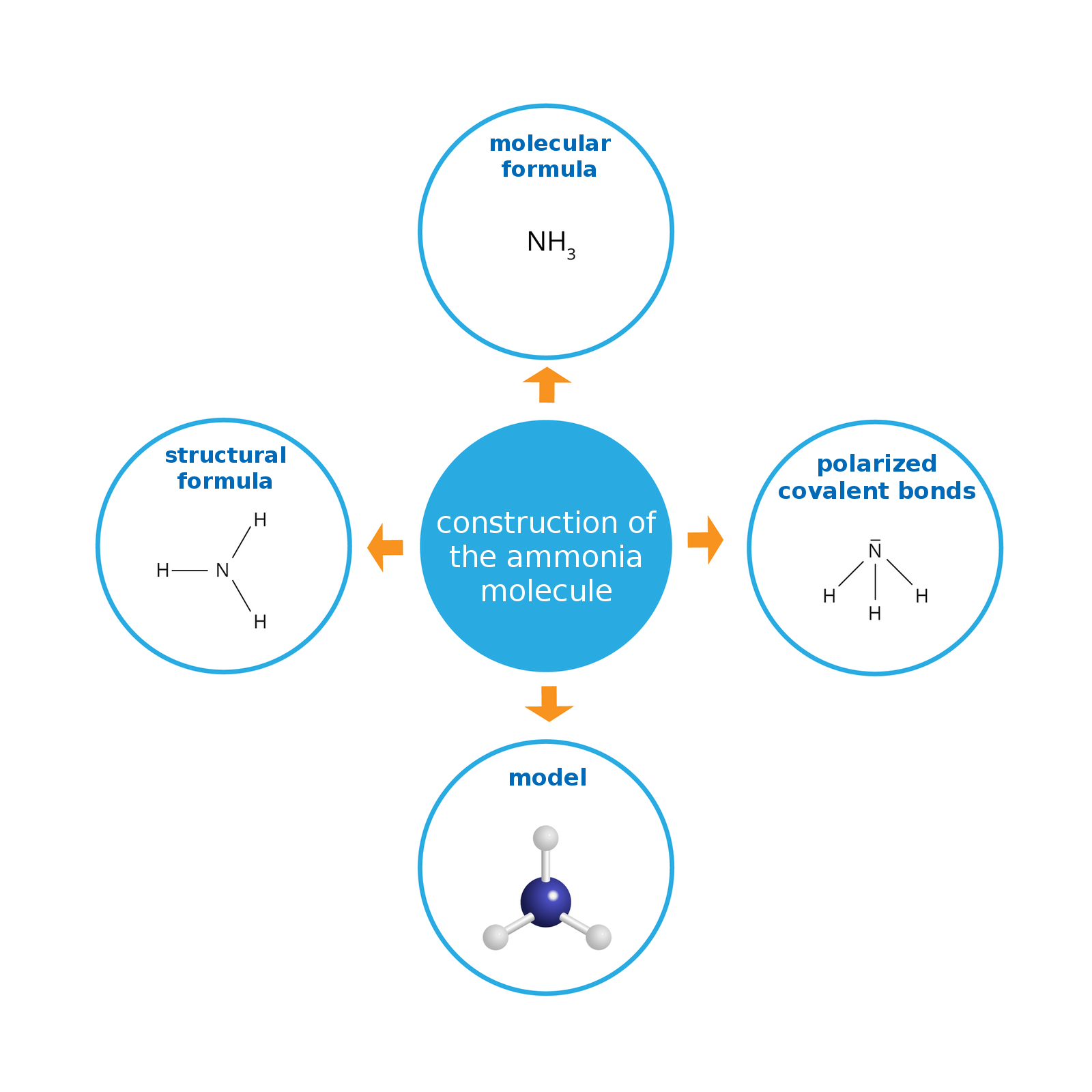
Structure and properties of ammonia
During the decomposition process of protein substances an unpleasant odor is released. One of the products is well‑known hydrogen sulfide smelling of rotten eggs. Another resulting gas with an equally unpleasant smell is ammonia – an inorganic compound with the formula .
ammonium chloride NHIndeks dolny 44Cl (s),
sodium hydroxide NaOH (solution 20%),
phenolphthalein solution
separatory funnel
round‑bottom flask
flat‑bottom flask
stand with a paw
crystallizer
3 rubber plugs with holes for tubes
screw clamp
2 flexible rubber tubes
test‑tube
splint
universal paper
4 laboratory tubes
The laboratory equipment should be prepared as per the diagram.
I. Preparation of ammonia from ammonium chloride:
Place ammonium chloride (up to 1/3 of its height) in a flat bottom flask, stop the flask. Pour the sodium hydroxide solution into the separatory funnel and attach it to the plug.
Slowly introduce the sodium hydroxide solution into the flask with ammonium chloride
Check the evolution of gas with a wetted indicator paper.
Collect gas into a test tube with a stopper attached to the second tube.
Clamp the screw clamp on the gas supply tube to the round‑bottom flask.
Then disconnect the flask with the separatory funnel from the set.
II. Ammonia solubility in water:
Pour phenolphthalein solution into the crystallizer.
Place the crystallizer under the round bottom flask with ammonia and immerse the end of the labolourin tube in the phenolophtheline solution.
Observe the changes and write down the observations.
III. The smell and flammability of ammonia:
Remove the cap from the gas tube.
Carefully smell the gas released from the test tube, and then insert the burning splint into the test tube.
Observe the changes and write down the observations.

Preparation of ammonia from ammonium chloride: NHIndeks dolny 44Cl + NaOH → NHIndeks dolny 33 + NaCl + HIndeks dolny 22O
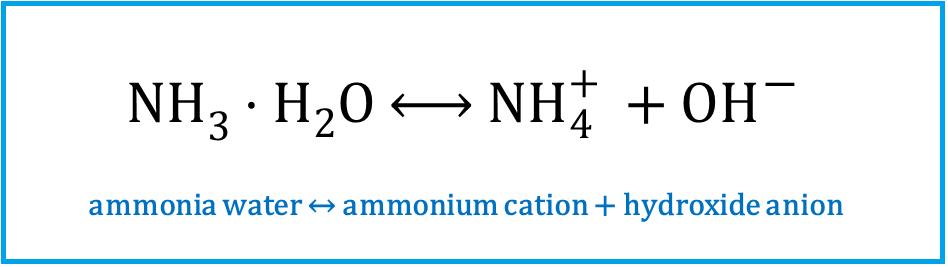
Reaction in water: NHIndeks dolny 33 + HIndeks dolny 22O → NHIndeks dolny 44Indeks górny ++ + OHIndeks górny --
Write the research question and hypothesis before doing the experiment “Testing the electrical conductivity of an aqueous ammonia solution”. Furthermore, write your observations and conclusions.
beaker,
device – electrical conductivity meter,
distilled water,
ammonia solution.
Fill the beaker with the aqueous solution of ammonia.
Immerse the electrodes of the conductivity meter in the liquid.
Observe the changes – pay attention to whether the light bulb has lighted up. Take the observation conclusions and write them down below.
Mark true statements.
- The ammonia solution conducts electricity.
- Ammonia is a flammable, soluble substance with a neutral pH.
- Ammonia, despite the unpleasant smell, is used in the food industry, for example in baking powder.
- Ammonia may be used for foliar fertilization of plants.
Summary
Ammonia is an inorganic compound with the formula NHIndeks dolny 33
It is a gas with an unpleasant odor.
The ammonia solution conducts electricity.
Keywords
ammonia, inorganic compounds, amines, amino acids
Glossary
amoniak – nieorganiczny związek chemiczny azotu i wodoru o wzorze NHIndeks dolny 33