Solubility of substances - tasks
that solubility is the number of grams of substance that can be dissolved in 100 g of solvent at a given temperature and under constant pressure;
that the graph showing the dependence of the solubility of a given substance on the temperature is called the solubility curve;
that as the temperature increases, the solubility of gas in water decreases, whereas solids – usually increase.
to interpret solubility curves to determine the solubility of a substance or type of solution (saturated, unsaturated);
to calculate the mass of substance needed to make its solution saturated at a specific temperature;
to calculate the mass of the components (solute and solvent) of the saturated solution at the set temperature.
Reading and interpretation of data from the solubility plot
Based on the solubility plot, we can determine how many grams of the substance will dissolve in 100 g of water and create a saturated solutionsaturated solution at a given temperature. We can also predict whether the given mass of substance can dissolve in a given mass of water.
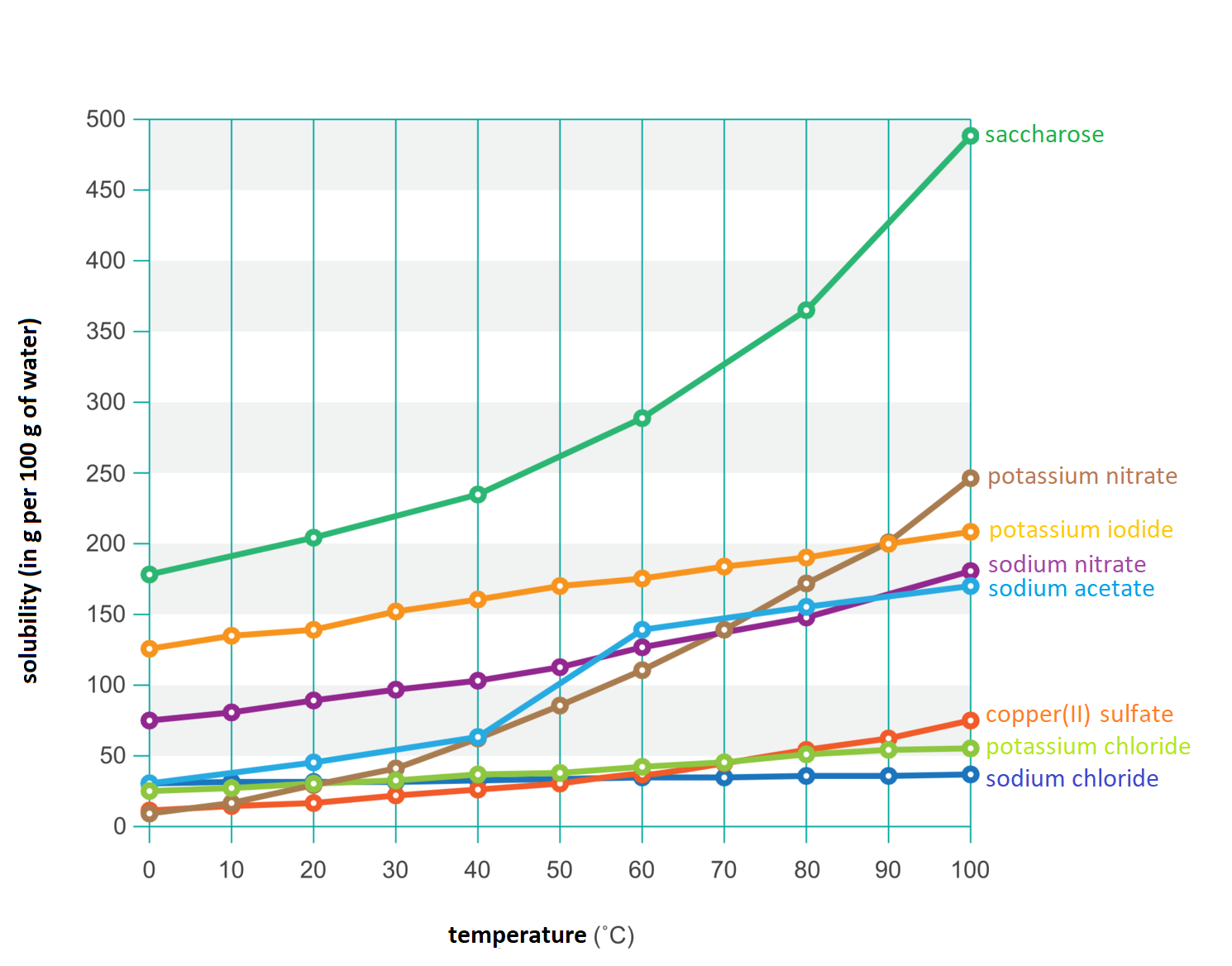
How many grams of sodium nitrate should be dissolved in 100 g of water to obtain a saturated solution at 40°C?
Solution
Place answer in the field below.
To obtain a saturated solution of sodium nitrate at 40°C, dissolve ............ g of this compound in 100 g of water.
Calculate the mass ratio of sodium acetate and water in the saturated solution at 0°C.
Solution
Place answers in the fields below.
The mass ratio of sodium acetate to water in a saturated solution of sodium acetate in water is ............:............, which means that for every ............ g of water there is ............ g of sodium acetate.
Check at what temperature the saturated solution of potassium nitrate contains 169 g of this substance and 100 g of water.
Solution
Place answer in the field below.
Saturated solution of potassium nitrate contains 169 g of this substance and 100 g of water at ............°C
Check that 40 g of potassium nitrate can be dissolved in 100 g of water at 20°C.
Solution
Place answer in the field below.
You can dissolve 40 g of potassium nitrate in 100 g of water at 20°C. Is this true? ............(Yes or No)
Calculation of the amount of a substance that can be dissolved in a given amount of water
From the graph solubility curvesolubility curve we can read how many grams of substance should be dissolved in 100 g of water to obtain a solution saturated at a certain temperature. By making further calculations, we get information about the mass of the substance that can be dissolved in any mass of water.
Calculate how many grams of sodium chloride should be weighed so that after dissolving it in 250 g of water at 90°C, obtain a saturated solution of this substance.
Solution
Place answer in the field below.
Weigh ............ g of sodium chloride to give a saturated solution of this substance after dissolving it in 250 g of water at 90°C.
Check that the sodium chloride solution, which contains 74 g of this substance in 200 g water at 50°C, is a saturated solution.
Solution
Place answers in the fields below.
............ (Yes or No), a sodium chloride solution that contains 74 g of this substance in 200 g water at 50°C, ............ (is or isn't) a saturated solution.
Calculation of the amount of substance contained in a given mass of saturated solution
Knowing the solubility of a substance in water, we can easily determine the mass ratios between a solute and a solvent in a saturated solution. This in turn allows us to calculate the amount of substance present in any mass of saturated solution.
Calculate the mass of the potassium chloride solution saturated at 60°C if 100 g of water was used to make it.
Solution
Place answer in the field below.
The weight of the solution is ............ g.
Calculate the mass of the sugar solution saturated at 20°C if 150 g of water was used to make it.
Solution
Place answer in the field below.
The weight of the saturated sugar solution is ............ g.
Calculate how many grams of copper(II) sulphate and water are found in 241.4 g of a saturated solution of this substance at 20°C, if its solubility at this temperature is 20.7 g in 100 g of water.
Solution
Place answers in the fields below.
In 241.4 g of a saturated solution of copper(II) sulfate at 20°C, ............ g of solute and ............ g of water (solvent).
Complete the text using the solubility curve graph.
To obtain a saturated solution of potassium nitrate at 60°C, it should be dissolved in 150 g of water ............ g this substance. If this solution is heated to 70°C, we can still dissolve ............ g of potassium nitrate and get a saturated solution. However, when the whole is cooled to 10°C, ............ g of potassium nitrate crystallizes out.
Prepare the task to calculate the amount of substance present in the mass of the chosen saturated solution. Give four answers to choose from, including the correct one.
Question: ...
- ...
- ...
- ...
- ...
Summary
Based on the knowledge of the solubility of a substance, you can: determine the amount of substance that will dissolve in 100 g of water and any mass of water, creating a saturated solution at a given temperature, assess whether the given amount of substance can dissolve in a given mass of water.
Find information on the internet about the sugar content in 1 liter of a popular cola drink. Assume that 1 liter of this drink weighs 1000 g. Then calculate how many grams of sugar should be added to obtain a saturated sugar solution at 20°C.
Keywords
solubility curve, solubility, saturated solution, unsaturated solution, concentrated solution, diluted solution
Match the pairs: English words with Polish definition.
wykres przedstawiający zależność rozpuszczalności danej substancji od temperatury, roztwór, który w danej temperaturze zawiera maksymalną ilość substancji rozpuszczonej, a dodana kolejna do niej porcja substancji nie ulega rozpuszczeniu, roztwór, który zawiera co najmniej kilkakrotnie mniej substancji rozpuszczonej niż roztwór stężony, określa maksymalną ilość substancji, jaka może rozpuścić się w 100 g rozpuszczalnika w danej temperaturze i pod stałym ciśnieniem, roztwór, w którym ilość substancji rozpuszczonej jest taka sama jak w roztworze nasyconym lub niewiele mniejsza, roztwór, który w danej temperaturze nie zawiera maksymalnej ilości substancji rozpuszczonej i w którym można rozpuścić dodatkową porcję tej substancji
| solubility curve | |
| solubility | |
| saturated solution | |
| unsaturated solution | |
| diluted solution | |
| concentrated solution |
Glossary
krzywa rozpuszczalności – wykres przedstawiający zależność rozpuszczalności danej substancji od temperatury
rozpuszczalność – określa maksymalną ilość substancji, jaka może rozpuścić się w 100 g rozpuszczalnika w danej temperaturze i pod stałym ciśnieniem
roztwór nasycony – roztwór, który w danej temperaturze zawiera maksymalną ilość substancji rozpuszczonej, a dodana kolejna do niej porcja substancji nie ulega rozpuszczeniu
roztwór nienasycony – roztwór, który w danej temperaturze nie zawiera maksymalnej ilości substancji rozpuszczonej i w którym można rozpuścić dodatkową porcję tej substancji
roztwór rozcieńczony – roztwór, który zawiera co najmniej kilkakrotnie mniej substancji rozpuszczonej niż roztwór stężony
roztwór stężony – roztwór, w którym ilość substancji rozpuszczonej jest taka sama jak w roztworze nasyconym lub niewiele mniejsza