Temperature and its relationship with the kinetic energy of molecules
Temperatura i jej związek z energią kinetyczną cząsteczek
to differentiate between temperaturetemperature and heatheat,
give the definition of absolute temperature scaletemperature scale,
convert temperature values from one scale to another.
Answer the following questions and then compare your answers with the model ones.
What is temperaturetemperature?
What determines the temperaturetemperature of the body?
How do particlesparticles of the body behave when the body temperaturetemperature increases?
Is there the lowest possible body temperaturetemperature?
What are temperaturetemperature scales for?
How is the absolute temperature scalethe absolute temperature scale defined?
Define the Celsius temperature scaletemperature scale.
Compare the Celsius scale with the Kevin scale.
Define the concept of heatheat.
What is the difference between temperaturetemperature and heatheat?
Can we talk about heatheat even at very low temperatures?
Model answers
1. What is temperaturetemperature?
TemperatureTemperature is one of the basic physical quantities describing the state of the body. The temperature is related to the average kinetic energy of motionmotion and vibrations of all molecules forming a given system and it is a measure of this energy.
2. What determines the temperaturetemperature of the body?
In microscopic terms, the temperaturetemperature depends on the movement of the molecules of which the body is built.
3. How do particlesparticles of the body behave when the body temperaturetemperature increases?
The temperature rises when the energy of molecules in motionmotion also increases. The movement may be associated with the movement of the molecules (e.g. in a gas), with the vibrations of atoms and molecules (e.g. in a crystal).
4. Is there the lowest possible temperaturetemperature of the body?
Zero on the Kelvin scale, also called the absolute or the absolute zero, is the lowest possible temperature in nature and corresponds to the situation when all motions of the atoms and molecules of which matter is built stop. Then, the molecules have zero kinetic energy.
5. What are temperature scales for?
Temperature scales are ordered temperature values (taken as defined thermal states) presented in the form of a number and temperature unit.
Defining the temperature scaletemperature scale requires:
Assuming at least one temperaturetemperature as the basic point of the scale and assigning this temperature a specific value (e.g. at normal pressure, at 0°C water changes its state from liquid to solid and vice versa).
Determining the appropriate unit of temperaturetemperature.
6. How is the absolute temperature scalethe absolute temperature scale defined?
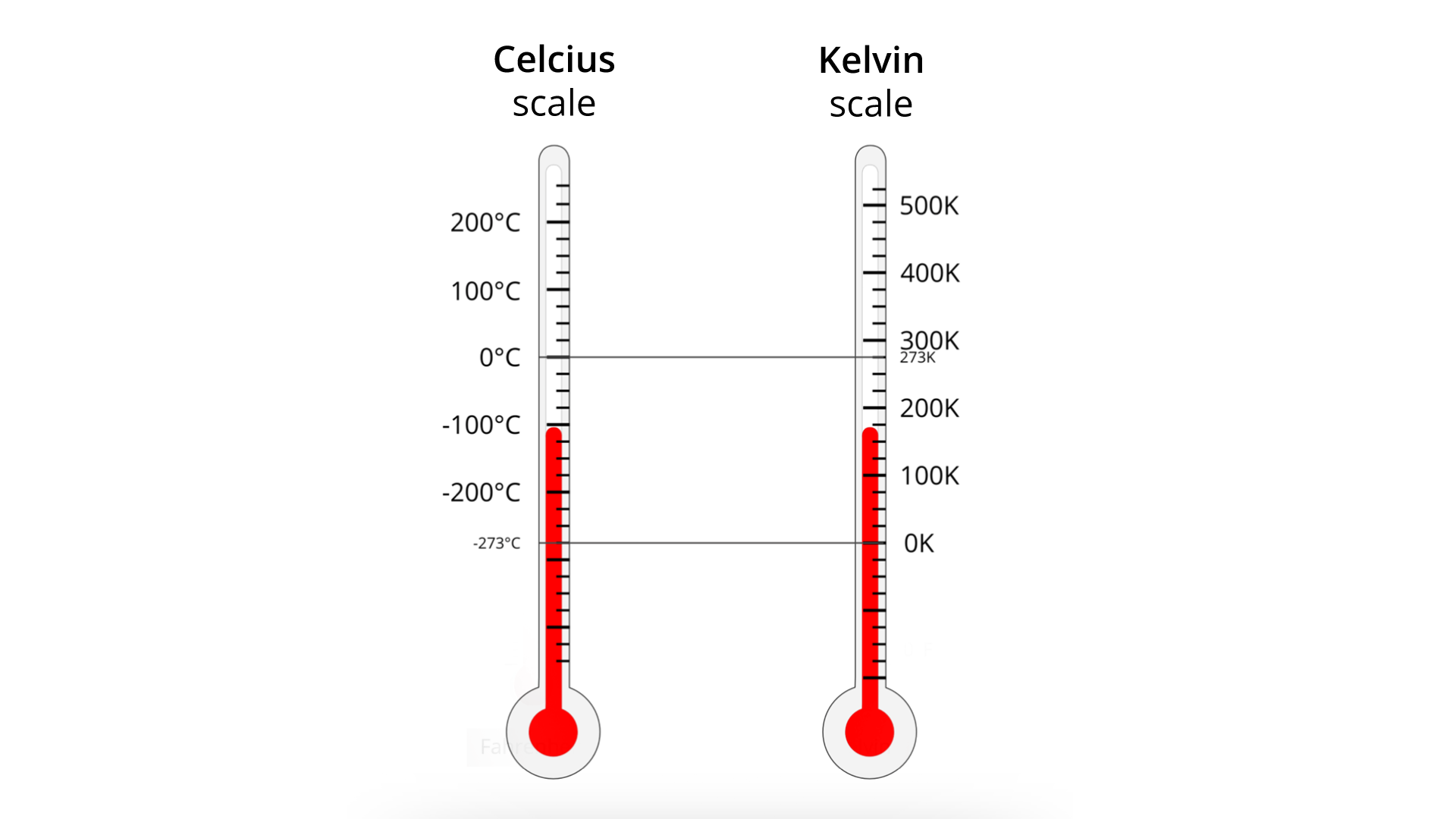
The Kelvin scale is the basic unit of temperaturetemperature in the SI system. In this scale the water triple point was assigned to the value which equals 273,15 K. The absolute zero temperature is assumed to be the zero value on this scale. The unit of temperature in this scale is Kelvin (1 K). The value of T was selected in such a way that between the temperature value TIndeks dolny kk in the Kelvin scale and the temperature value TIndeks dolny cc in the Celsius scale the following relationship holds:
7. Define the Celsius temperature scaletemperature scale.
The Celsius scale is based on two constant points: 0Indeks górny ooC – the melting point of the ice at normal pressure and 100Indeks górny ooC – the boiling point of water at the normal pressure. The unit of temperaturetemperature in this scale is one degree Celsius ().
8. Compare the Celsius scale with the Kevin scale.
One degree on the Celsius temperature scaletemperature scale is always equal to one Kelvin. The Kelvin scale is similar to the Celsius scale, and the only difference between them is to shift the numerical values in both scales in relation to each other by a constant value.
Click on the number then the description will appear.
9. Define the concept of heatheat.
HeatHeat in physics is one of the methods of transmitting internal energyinternal energy to the thermodynamic system by transmitting the energy of the chaotic movement of particles (atoms, molecules, ions). The second method to transfer internal energy is work.
10. What is the difference between temperaturetemperature and heatheat?
Temperature is a measure of the thermal state of the body (substance). HeatHeat is a form of energy that can be transmitted (given back or received).
11. Can we talk about heatheat even at very low temperatures?
Yes! If only bodies with different temperatures (even very low) are in contact with each other, then the flow of heatheat occurs.
Summary
The physical quantityphysical quantity called temperaturetemperature is related to the average kinetic energy of atoms and molecules - two bodies have the same temperature if the average kinetic energy of their atoms or molecules is the same. Bodies with higher temperature have a higher value of the average kinetic energy of their atoms and molecules.
On the Kelvin scale (also known as the absolute temperature scalethe absolute temperature scale) the temperaturetemperature is directly proportional to the average kinetic energy of atoms or molecules.
On the Celsius scale, the zero point is the temperaturetemperature at which water freezes, and 100 degrees is the temperature at which the water boils at normal atmospheric pressure.
Zero Kelvin (also referred to as absolute zero) is the lowest possible temperaturetemperature in nature; at this temperature the average kinetic energy of atoms and molecules is zero (atoms and molecules are in stillness).
The temperaturetemperature difference in the Celsius scale and the absolute scale is equal.
Exercises
Decide which sentences are true.
- If two bodies are in contact, heat always flows between them.
- The temperature determines if heat flows during the contact between two bodies.
- The temperature of a certain body increased from 200 K to 400 K. What follows from this statement is that the speed of the movement of the molecules inside the body has doubled.
- The temperature of a certain body increased from 200 K to 400 K. What follows from this statement is that the average kinetic energy of the movement of the molecules inside the body has doubled.
Express the temperature , on the Kelvin scale and calculate and .
Experiment
In a laboratory shop (or online) purchase a thermoscope.
Build a homemade thermometer. Use coloured alcohol as the liquid. Use your own temperature scale. Remember to define two reference points. What conditions must such points meet?
Indicate which pairs of expressions or words are translated correctly.
- temperatura - temperature
- ciepło - heat
- termometr - thermometer
- cząsteczki - particles
- skala temperatur - heat exchange
- energia wewnętrzna - heat exchange
- temperature
- ciepło
- motion
- thermometer
- ruch
- heat
- temperatura
- termometr
- particles
- cząsteczki
Glossary
ciepło
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: heat
wymiana ciepła
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: heat exchange
energia wewnętrzna
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: internal energy
ruch
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: motion
cząsteczki
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: particles
wielkość fizyczna
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: physical quantity
temperatura
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: temperature
skala temperatur
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: temperature scale
bezwzględna skala temperatur
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: the absolute temperature scale
termometr
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: thermometer
Keywords
heatheat
motionmotion
particlesparticles
temperaturetemperature
thermometerthermometer