Mixing of substance - structure of matter and diffusion
what properties matter has in various states;
what a mixture is;
on the basis of which criteria mixtures are classified;
what safety rules should be followed in the school chemical laboratory.
describe substance concentrations with examples;
explain the phenomenon of diffusion and contraction;
plan experiments confirming the granularity of matter structure;
Structure of the matter
Everything around us is mattermatter. It is made of particles (it has a granular structure). In the solid substance, the particles are arranged close to each other in a regular manner. In liquids the distances between the particles are usually larger and the particles are arranged irregularly. However, in gases the distances between irregularly arranged particles are the largest (which means at the same time that the interactions between particles are the smallest in this case).
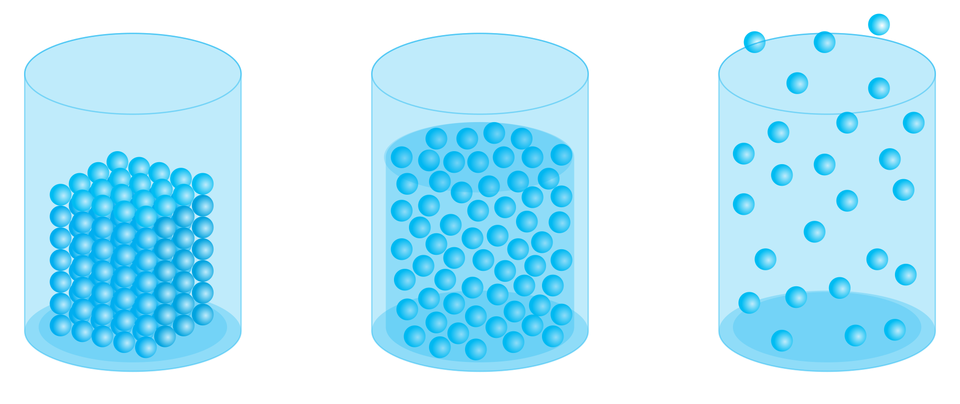
Plan an experiment that would confirm that the matter is made of particles.
Before you watch a movie or make an experiment „The process of mixing water with ethanol”, formulate a research question and hypotheses. During the sceening, pay attention to what happens with the liquid volume.

Film dostępny na portalu epodreczniki.pl
Nagranie filmowe przedstawia mieszanie się wody z etanolem. Do cylindra wlewamy 50 mililitrów etanolu, ethanol. Następnie wlewamy 50 mililitrów wody i mieszamy roztwór. W wyniku zmieszania 50 mililitrów jednej cieczy i 50 mililitrów drugiej cieczy w cylindrze powinniśmy otrzymać 100 mililitrów cieczy. W tym przypadku objętość cieczy w cylindrze wynosi 95 mililitrów. Całkowita objętość dwóch mieszanych cieczy nie jest równa sumie ich objętości.
Is the total volume of two mixed liquids equal to the sum of their volume?
Select one of the following hypotheses and then verify it.
The total volume of two mixed liquids will be equal to the sum of their volume.
The total volume of two mixed liquids will not be equal to the sum of their volume.
3 measuring cylinders,
glass rod,
water,
ethanol
Measure 50 cmIndeks górny 33 of water and ethanol in separate measuring cylinders.
Pour measured volume of water and ethanol into 100 cmIndeks górny 33 cylinder.
Mix components and read out the volume of the resulting mixture.
What happens if we mix equal volumes of peas and poppy seeds?
Select one of the following hypotheses and then verify it.
The total volume will not be equal to the sum of the volume of the individual components.
The total volume will be equal to the sum of the volume of the individual components.
3 measuring cylinders,
peas,
poppy seeds or buckwheat groats.
Measure 100 cmIndeks górny 33 of peas and buckwheat groats or poppy seeds in measuring cylinder.
Put measured volume of peas and buckwheat groats or poppy seeds into 250 cmIndeks górny 33 cylinder.
Mix thoroughly all components of the mixture.
The phenomenon of volume reduction when mixing liquids is called contractioncontraction. The reason for contraction is the possibility of stiffer packing of particles in a mixture of two liquids. The mechanism of contraction can be presented in a model way, using for example peas and poppy seeds.
What is diffusion?
Watch the gallery of photos. Think about what these have in common.
In which situations can you feel the intense aromas? Consider it and name examples of such circumstances or places.
What happens if we open the perfume bottle in the classroom?
The fragrance will spread and will be noticeable away from the bottle.
perfumes.
Place an open perfume bottle in the corner of the classroom and leave it for 5 minutes.
After a while the smell of perfume is noticeable in the whole class.
Perfumes consist of particles that by itself mix with the air and spread in it.
Can a substance by itself spread in another substance?
Substance can by itself spread in another substance.
balloon,
dropper,
box,
fragrance oil.
Inflate the balloon.
Add few drops of fragrance oil into balloon.
Tie a balloon and close it in the box.
After an hour open the box.
Fragrances of cosmetics, flowers or cooked and fried foods are spreading throughout the apartment. The air freshener slowly releases the pleasant fragrance that we feel in the room. The scent of hairspray after a short moment becomes noticeable away from the place of its spraying. The examples cited illustrate the process of spreading the substance fragrance by itself from the place where it is focused, to places where there is less or none smell, which leads to equalization of concentrations. This is the phenomenon of diffusiondiffusion.
Advection
The transmission of smell in the air does not always occur by itself, it can be caused by the phenomenon of advection (horizontal movement of molecules of the transported substance). It causes the inflow of air with different properties (e.g. with different temperature or humidity). The phenomenon of advection in combination with diffusion is called convection (vertical movement associated with heat movements, with movements caused by temperature difference).
There are following types of advection:
forced, e.g. draught in the room;
natural, caused by differences in the density of the medium due to temperature or concentration differences.
Diffusion of gases is quite slow process, for example, the shift of ethanol vapours in the air at a distance of 1m takes about 100,000s, which lasts for over 1 day! Thanks to the advection effect with almost imperceptible air flow (10 cm/s), the fragrance is transferred to a distance of 1 m in just 10 seconds!
A special type of diffusion is osmosis, which occurs in aqueous solutions, penetrating the semi‑permeable membrane separating two solutions of different concentration. Osmosis proceeds spontaneously, from a solution with a lower concentration of dissolved substance to a solution with a higher concentration, i.e. it leads to equalization of the concentrations of both solutions.
Summary
Matter is made of particles that are constantly in motion. Matter has a granular structure.
The most important phenomena confirming the discontinuity of matter are: diffusion, dissolution of solids in liquids, mixing of liquids, changes in the state of matter.
Diffusion in gases and liquids occurs faster than in solids.
Keywords
diffusion, contraction, matter, convection, advection
Glossary
dyfuzja – zjawisko polegające na samorzutnym mieszaniu się substancji, w taki sposób, że drobiny jednych substancji wnikają pomiędzy drobiny innej substancji; zachodzi z różną szybkością i prowadzi do równomiernego rozmieszczenia drobin
kontrakcja objętości – zjawisko zmniejszania się objętości roztworów podczas mieszania, np. wody i etanolu; spowodowane oddziaływaniem drobin i mieszających się substancji
materia – składa się z drobin, ma budowę nieciągłą – ziarnistą
osmoza – zjawisko polegające na samorzutnym mieszaniu się substancji, zachodzi w roztworach wodnych przez błonę półprzepuszczalną rozdzielającą dwa roztwory o różnym stężeniu do momentu wyrównania stężeń






