Oxygen: properties and application
how to plan the experiment confirming that the air is a mixture;
what are symbols of chemical elements and how to use them;
how the equations of chemical reactions are noted dow;
how to recognize basic laboratory equipment and how to use it, how to apply the safety rules in a school chemical laboratory in practice.
to indicate oxygen properties;
to give examples of the use of oxygen in everyday life.
Properties of oxygen
Under normal conditions (0°C, 1013,25 hPa) oxygen is colourless and odourless gas that creates diatomic molecules.
In the upper atmosphere, under the influence of ultraviolet radiation, ozone is formed, a blue gas whose molecules are made up of three oxygen atoms – it is therefore an allotropic form of oxygen. It occurs in nature: it is formed mainly after a storm due to lightning discharges; it also occurs in coniferous forests.
Ozone has strong antiseptic properties. It destroys microbes, bacteria, viruses and fungi; it purifies the air and removes unpleasant odours. Its action is very strong but short‑term. Due to these properties, ozone, produced in laboratories, is used in medicine, rehabilitation, cosmetology and many industries.
Compare information about oxygen and its allotropic form – ozone.
Ozone
Ozone molecule
Three atoms of oxygen
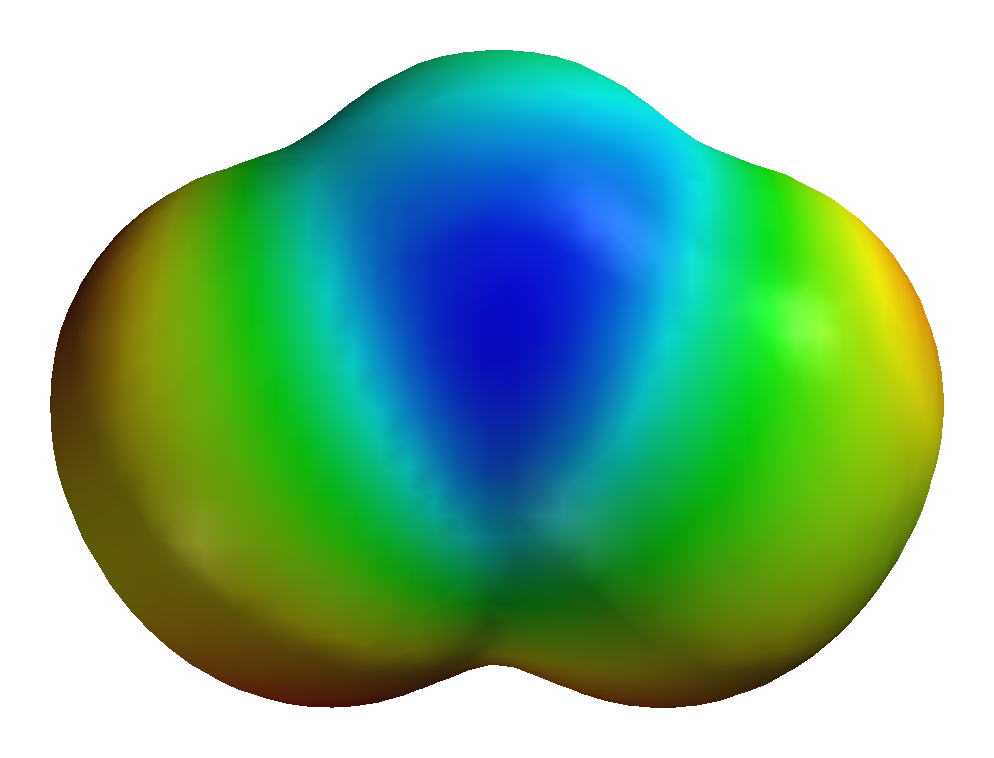
Molecular formula
O3
State of matter
blue gas, dark blue liquid, dark violet solid
Metallic properties
Non-metal
Odour
Sharp, fresh – it is a smell of air after a storm
Density
2.1 kg/m³
Melting point
−193°C
Boiling point
−112°C
Solubility in water
Good
Combustibility
Incombustible, supporting combustion
Oxygen application
Oxygen is widely used in medicine and various industries. Look at the interactive illustration and memorise where and what purpose oxygen is used.
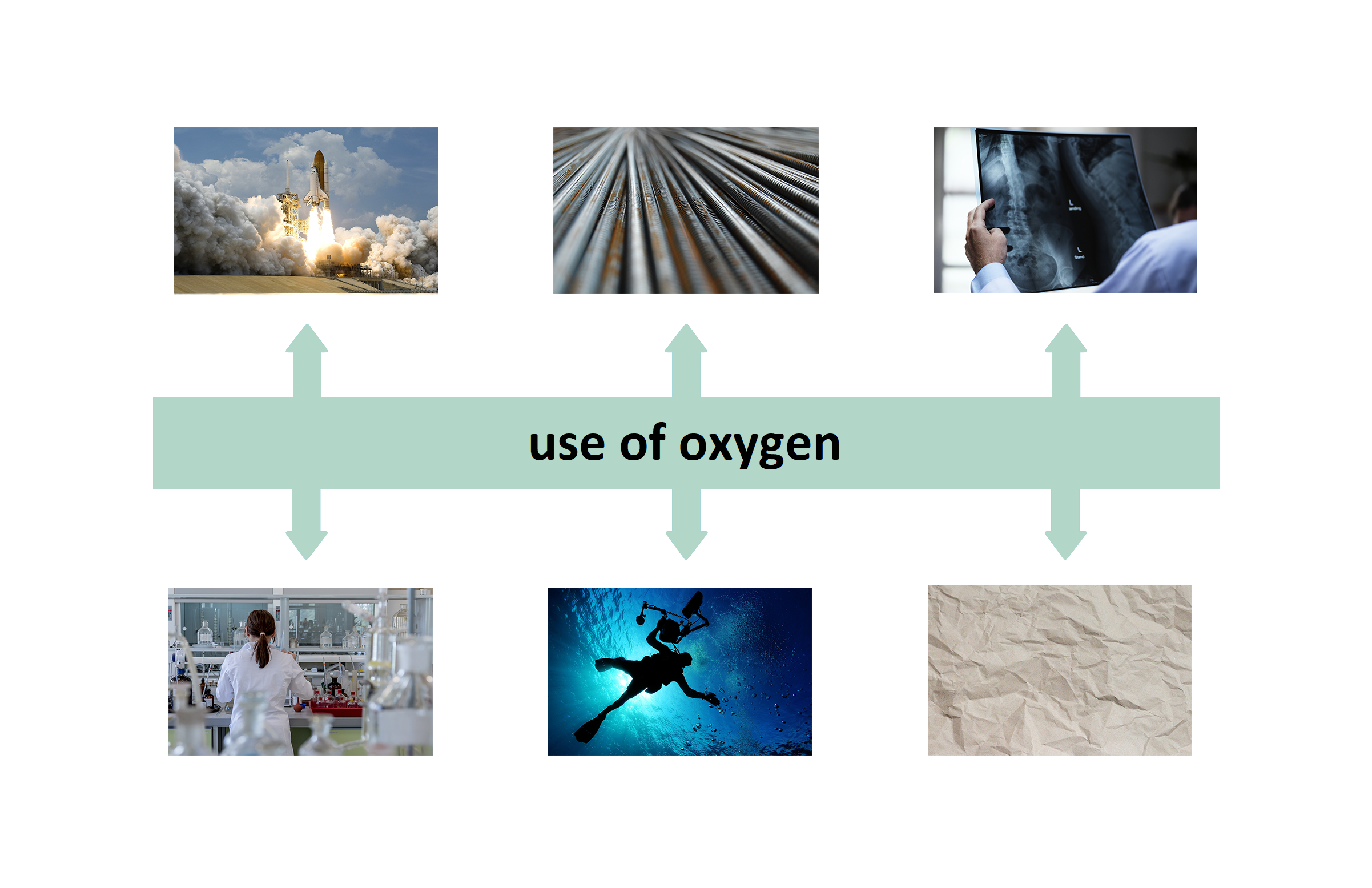
1. As a fuel oxidizer in rockets and space shuttles (liquid)
2. to produce high temperatures in metal welding and cutting torches
3. in oxygen therapy, for example in the case of carbon monoxide poisoning
4. in hyperbaric chambers, for the treatment of serious skin damage
5. as part of a mixture in bottles for divers, aplinists and cosmonauts
6. for bleaching of (e.g.) paper, to asepticize operating rooms in hospitals and to disinfect water (ozone)
7. Use of oxygen
Select the affirmative answer if all of the drawn elements describe the proper application of gases.
|
ozone
|
formed as a result of the photosynthesis process
|

|
|
oxygen
|
used during treatment, e.g. in hyperbaric chambers
|
|
|
oxygen
|
used in metallurgy, e.g. for steel production
|

|
|
ozone
|
produced during lightning discharges
|

|
|
ozone
|
used in cosmetology, e.g. for moisturizing the skin
|

|
|
ozone
|
used to destroy saprophytes while cleaning carpets, upholsterings
|
|
|
oxygen
|
used for disinfection, e.g. in swimming pools
|

|
|
ozone
|
used in dentistry, e.g. to treat tooth decay
|

|
Select true statements.
- Ozone is generated during atmospheric discharges.
- Oxygen is formed as a result of the photosynthesis process.
- Oxygen density is higher than ozone.
- Ozone molecular formula is O2.
Summary
Oxygen is the most widespread element in nature.
Oxygen is a colourless, odourless gas, slightly soluble in water, very chemically active.
Allotropic oxygen form is ozone.
Ozone occurs naturally but can be obtained in the laboratory.
Ozone and oxygen are used in medicine, cosmetology, rehabilitation and many types of industry.
Keywords
Oxygen, properties of oxygen, application of oxygen, ozone, allotrope, air
Glossary
spalanie – reakcja z tlenem, której towarzyszy wydzielanie ciepła i światła
odmiana alotropowa – odmiana tego samego pierwiastka chemicznego w tym samym stanie skupienia, różniąca się właściwościami fizycznymi i chemicznymi. Odmiany takie mogą różnić się między sobą np. liczbą atomów w cząsteczce.