Air - composition and properties pt 1
how to determine and distinguish physical and chemical transformations;
what criteria are adopted for the classification of types of chemical reactions;
what are symbols of chemical elements and how to use them;
how to write equations of chemical reactions;
how to recognize basic laboratory equipment and how to use it, how to apply the safety rules in a school chemical laboratory in practice.
to provide evidence for the existence of air;
to plan and carry out experiments confirming that the air is a mixture;
to describe the composition and properties of air;
to safely use laboratory equipment and chemical reagents.
Does air exist?
What phenomena indicate the existence of air?
Suggest experimental evidence indicating the presence of air.
What indicates the presence of air in these photographs? Write down your suggestions by clicking in the white round boxes.

Conduct your own experiment and note down observations and conclusions.
Is there air in the glass and the syringe that are empty?
Select one of the hypothesis and then verify it.
There is no air in the „empty” glass and in the „empty” syringe. There is air in the „empty” glass and in the „empty” syringe.
syringe,
a glass,
bowl,
water,
paper.
Take the syringe and pull the plunger all the way down.
Close the mouth of the syringe and press the plunger.
Observe the changes.
Pour water into the bowl, preferably previously coloured.
Crease the sheet of paper and place in a glass.
Turn the glass upside down and put it into a bowl of water.
Observe the changes.
What is contained in the air?
During the formation of the Earth's crust and subsequent processes taking place on the Earth, the composition of our atmosphere, that is the gas coating, has changed, but for 200 million years it has already become permanent.
Find information on what the earth's atmosphere is and what it consists of on the Internet, the textbook and the e‑textbook. Write down the names of the five layers of the atmosphere.
Before you watch the movie „Testing the composition of the air”, write down the research question and hypothesis. Consider what indicates that the air is or is not a mixture of gases? Write observations and conclusions.

Film dostępny na portalu epodreczniki.pl
Film pokazuje eksperyment - z czego składa się powietrze, air composition test. Będziesz potrzebować: świeczka - podgrzewacz, miska z zabarwioną na niebiesko wodą, zapalniczka, zlewka. Zapalamy świeczkę i kładziemy na wodzie. Przykrywamy zlewką. Płomień świeczki gaśnie. Woda z miski przedostaje się do zlewki.
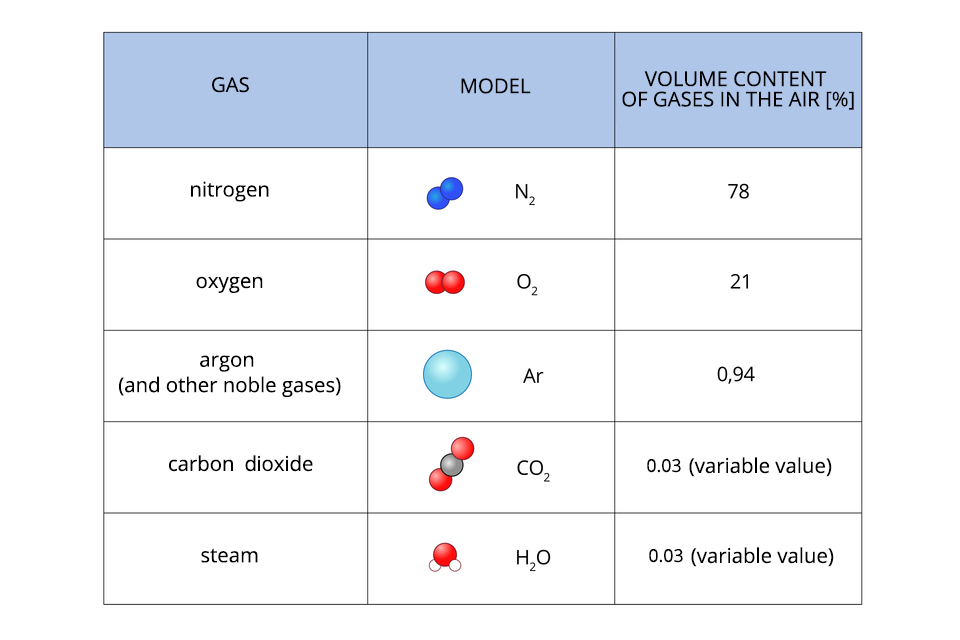
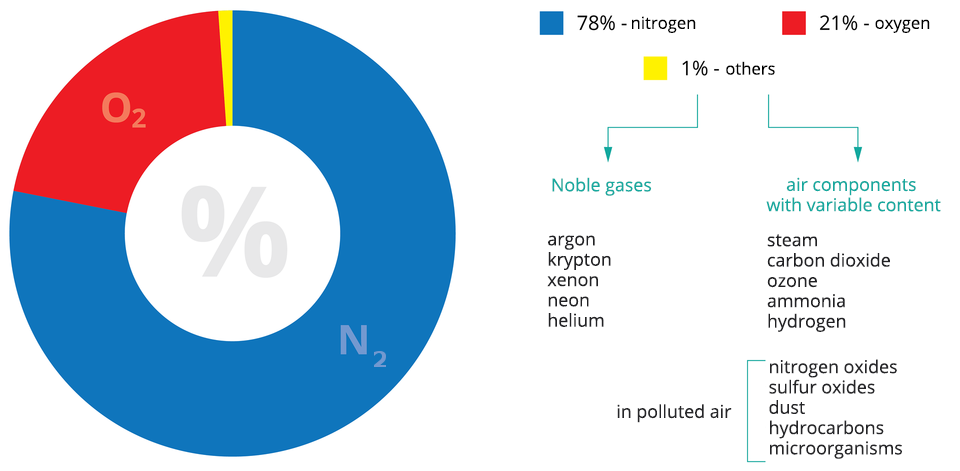
The air is mainly nitrogen, oxygen and a small volume of noble gases. Air components, whose content varies depending on the climate, seasons or day, include: water vapour, carbon dioxide, ozone and pollution.
Air/ component of the air | Colour | Density | Melting point | Boiling point |
air | colourless | 1.30 | -213 | -190 |
nitrogen | colourless | 1.25 | -210 | -196 |
oxygen | colourless | 1.43 | -219 | -183 |
argon | colourless | 1.78 | -189 | -186 |
Select correct statements.
- Nitrogen is the main component of the air
- Air is a heterogeneous mixture of different substances
- The air is slightly blue, hence the blue colour of the sky.
- The air contains about 1/5 of the oxygen volume
Summary
The air is a homogeneous mixture of colourless and odourless gases.
The main components of the air are: nitrogen (78%), oxygen (21%), argon and other noble gases (0.94%), carbon dioxide and water in the form of steam.
Keywords
air, oxygen, nitrogen, noble gases, atmosphere, homogeneous mixture
Glossary
powietrze – jednorodna mieszanina różnych substancji, głównie gazów, bez barwy, smaku i zapachu, stanowiąca atmosferę ziemską