Plaster mortar and application of gypsum rock
that the outer layer of the earth's crust form various rocks and minerals;
how to write patterns and create hydrate names;
how to interpret differences in the properties of hydrates and anhydrous substances.
describe the hardening process of gypsum mortar and record the appropriate reaction equation;
discuss the use of gypsum rock;
justify why calcined gypsum is used in medicine.
Before you watch the demonstration or conduct the experiment „Preparation of gypsum mortar and testing the rate of its hardening”, write down the research question and hypotheses. Also note observations and conclusions from the experiment.
How is gypsum mortar obtained and what factors affect its hardening?
Gypsum mortar quickly binds water.
calcined gypsum,
beaker with water,
porcelain evaporating dish,
spoon,
glass rod,
matchbox or other mould.
Pour 3–4 spoons of calcined gypsum into evaporating dish.
Gradually add water, stirring the contents of the vessel with a glass rod, until obtaining the consistency of thick cream.
One half of the obtained gypsum mortar should be placed in a matchbox or other mould, and the other half should remain in the evaporating dish.
After several minutes, check the hardness of the mortar in the evaporating dish, and leave the cast for a few hours.
After mixing water with calcined gypsum in appropriate proportions, the grey paste with the consistency of thick cream is formed. The resulting mixture, called gypsum mortar, hardens quickly. Gypsum mortar binds water. What is the name of this property? The hardening process of the mortar is exergonic and can be described with the following reaction equation:
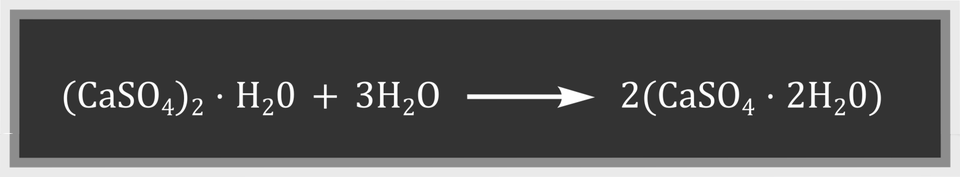
The hardening of gypsum mortar occurs under the influence of water, which is why the mortar is called a hydraulic lime mortar. The process occurring during hardening of gypsum mortar is the reverse calcination of a gypsum flower.
Due to the time of hardening of the mixture of calcined gypsum and water, there are several types of gypsum mortars that have different composition, which is why they are widely used in construction industry.
The building mortar hardens the quickest, after about 10 minutes of mixing it with water. Filler needs approx. 30 minutes. On the other hand, plaster mortar needs long‑time of water binding (including plaster), and hardening depends on the thickness of the layer and can take one to several hours.
Consider how can you distinguish gypsum rocks from calcareous rock?
Application of gypsum rock
Applications of gypsum are very diverse, especially in construction, where it is used to the formation of gypsum mortar, prefabricates, self‑levelling floors, for the production of cement and plasterboards, so‑called gypsum boards, sheets consisting of gypsum protected by cardboard. Gypsum is an ecological material, friendly to humans. Easily absorbs excess dampness from the atmosphere, and when the air in the room is dry, it gives off excessive dampness.
Gypsum is also used in the ceramic industry for the production of moulds. It is also used for the production of paints and varnishes. In agriculture it is used as a fertilizer and soil fertilizer, and in the food industry – for clarification of wines. A special application of gypsum is in medicine, where purified gypsum varieties are used, so‑called surgical and dental plaster.
Fracture fixation is known since ancient times. Wooden boards and ribbons of material impregnated with starch, resin, wax, egg whites and even lime were used for this purpose. Gypsum dressings arrived from Arabia to Europe in the eighth century. These did not have too many followers. This method was commonly used at the beginning of the ninth century when the powdered and calcined gypsum was used to immobilize the limbs.
Why is calcined gypsum used for immobilizing limbs instead of calcined lime?
Significant quantities of gypsum are used to create artistic products (modelling gypsum), such as gypsum figurines, masks, stucco, castings. In sculpting, alabaster is a particularly valuable material. It is used to manufacture, among others vases, cups, trays, candlesticks, ashtrays, lamp shades, stuccos and sculptures.
Gypsum karst
The karst processes and forms of gypsum karst are rare phenomena. This is caused by relatively small deposits of gypsum in the Earth's crust, as opposed to the amount of limestone. The karst processes of gypsum depend on dissolving by water, which, unlike limestone, does not have to contain carbon dioxide. This is possible because gypsum and anhydrite are moisture absorbent and moderately soluble in water. It also means that forms of gypsum karst are very unstable and relatively young compared to the limestone karst. These can be found in various parts of the world, also in Poland. In our country, these mainly occur in Ponidzie. Skorocicka Cave next to the Busko‑Zdrój is the largest Polish gypsum cave in the country. However, the most unusual gypsum cave is the Cave of the Crystals in the Mexican state of Chihuahua.
Cave of the Crystals, so‑called Sistine Chapel of Crystals, was discovered accidentally in 2000 by tunnelling miners.
This cave is famous due to the largest gypsum specimens discovered so far (many of them are huge, up to 15 meters in length, and weight up to 55 tons). The crystals in this cave are also one of the largest minerals in the world. The grotto has extreme climatic conditions, i.e. the temperature is up to 65.5°C, and the air humidity is almost 100%. For this reason, its exploration is possible only in special protective suits, equipped with masks connected to oxygen tanks. Otherwise, people should not stay in the cave for longer than 10 minutes.
Look for information about the Skorocicka Cave in the vicinity of Busko‑Zdrój and the Crystal Cave in Naica, also known as the Crystal Sistine Chapel. Answer the question: „Why nature was the most perfect creator in terms of exhibits obtained from gypsum?”.
Solve the crossword and explain the meaning of the answer you received.
- Anhydrous gypsum
- Sedimentary rock along with limestone and chalk.
- (CaSO4)2 • H2O, is the formula of … gypsum
- The dissolution process of rocks by surface and underground waters
- A chemical compound that is part of all hydrates
- Noble, fine-crystalline gypsum type
- The name of the water that is part of the hydrate structure is "Water of ..."
| 1 | ||||||||||||||||||||
| 2 | ||||||||||||||||||||
| 3 | ||||||||||||||||||||
| 4 | ||||||||||||||||||||
| 5 | ||||||||||||||||||||
| 6 | ||||||||||||||||||||
| 7 |
Match the pairs: English words with Polish definition.
siarczan(VI) wapnia—woda(1/2), mieszanina gipsu palonego i wody, twardniejąca pod wpływem wiązania wody, woda krystalizacyjna (hydratacyjna), siarczan(VI) wapnia—woda(2/1), w chemii nieorganicznej, sole, które zawierają cząsteczki wody wbudowane w sieć krystaliczną
| hydrates | |
| water of crystallization (hydration) | |
| crystalline gypsum | |
| burned gypsum | |
| plaster mortar |
Summary
Gypsum rock contains calcium sulphate in its composition.
As a result of roasting a crystalline gypsum at about 120°C, gypsum is formed and anhydrous calcium sulphate is formed above 180°C.
Crystal gypsum and calcined gypsum are examples of hydrated salts (hydrates). Anhydrite is an example of anhydrous salt.
Hydrates are unstable and, during heating, go into anhydrous or lower hydration salts.
Plaster mortar is an example of hydraulic mortar.
Gypsum rock is used in construction, medicine, agriculture, in the industry: ceramic, food, chemical. They are also a valuable material in the hands of artists.
Keywords
gypsum, calcined gypsum, anhydrite, calcium sulphate, hydrates, hydraulic mortar
Glossary
hydraty – w chemii nieorganicznej, sole, które zawierają cząsteczki wody wbudowane w sieć krystaliczną
woda krystalizacyjna (hydratacyjna)
gips krystaliczny – siarczan(VI) wapnia—woda(1/2)
zaprawa gipsowa – mieszanina gipsu palonego i wody, twardniejąca pod wpływem wiązania wody