The structure of the atom and the location of the element in the periodic table
that the elements are ordered in the periodic table according to the increasing atomic number;
that electrons in the atom are distributed on the shells;
that the record showing the distribution of electrons in the atom is called the electronic configuration;
that electrons on the last shell are called valence electrons.
determine the number of valence electrons in the atoms of the elements located in the groups: 1, 2 and 13–18;
determine the number of shells in the atoms of elements based on the period number;
that elements belonging to one group have similar properties, and elements from the same period do not show characteristic similarities.
Structure of the atom vs. number of the group of the element
Periodic tablePeriodic table next to the necessary atomic numbers and symbols of elements can also contain other information.
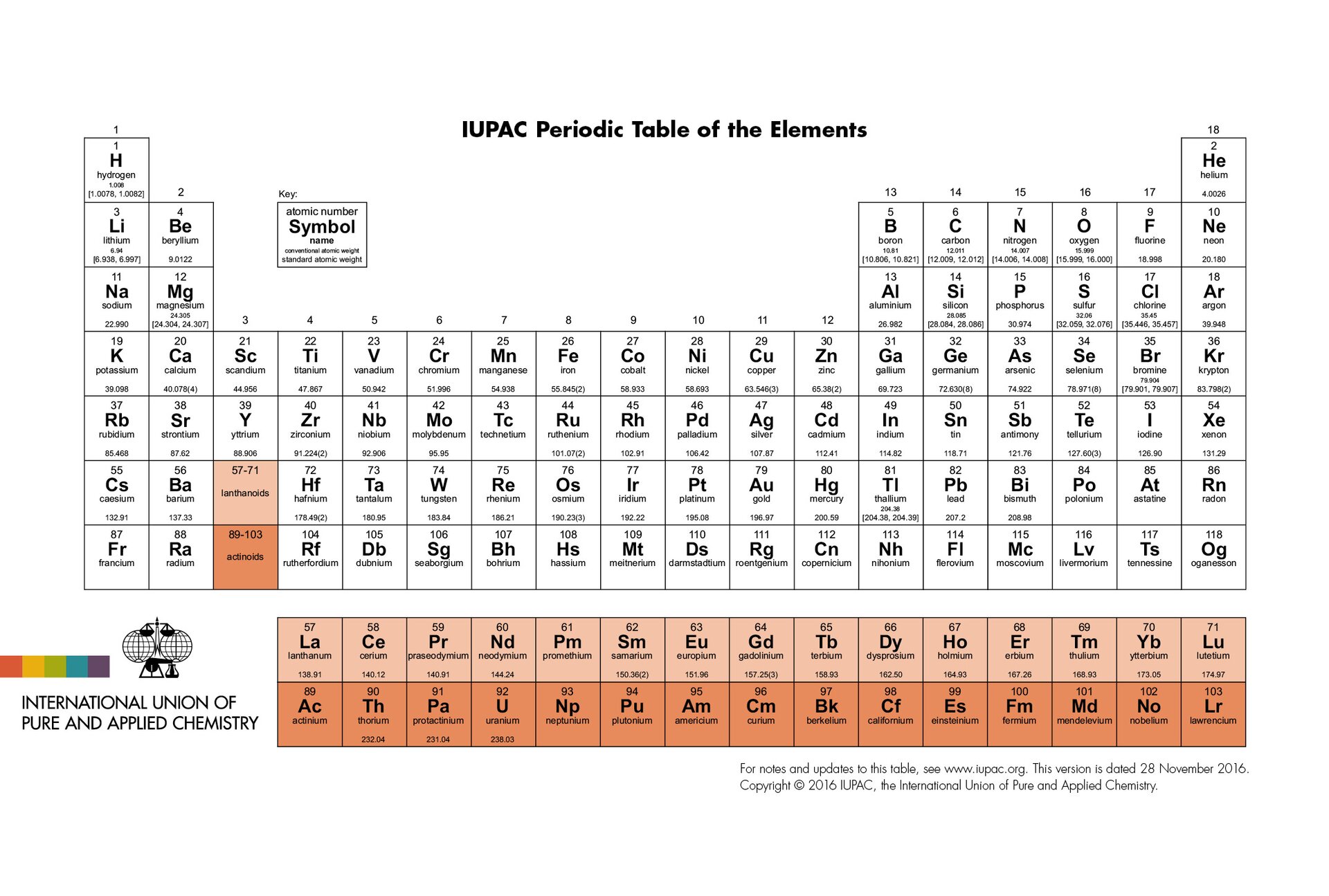
Search in the periodic table for the sets of elements whose atoms have the same number of valence electrons. Is there a regularity between the number of valence electrons and the position of the element in the periodic table? Write down the answer.
After careful analysis of the data contained in the periodic table of elements, it can be noticed that within some groups the elements have the same number of electrons on the last shell. This observation concerns groups: 1, 2, 4, 13, 14, 15, 16, 17 and partly 18. In groups: 3 and 5‑12 the number of these electrons in atoms of elements varies.
Group | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. |
Number of electrons on the last shell | 1 | 2 | various | 2 | various | various | various | various | various |
Group | 10. | 11. | 12. | 13. | 14. | 15. | 16. | 17. | 18. |
Number of electrons on the last shell | various | various | various | 3 | 4 | 5 | 6 | 7 | helium |
Find the sets of elements whose atoms have the same number of electron shells in the periodic table. Is there a certain relation between the number of shells in the atom and the location of an element in the periodic table?
On the table of elements taking into indicating electron configurations, it is clearly visible that the elements whose atoms are made of the same number of shells are in the same period, in addition the number of these shells is equal to the number specifying the period number. This observation allows us to draw a general conclusion about the structure of atoms of elements, namely that the number of shells in the atoms of the element equals the number of the period in which the element is located.
The structure of atoms and the location in the periodic table of elements
There is a connection between the structure of the atom and the location of an element in the periodic table. Number of the period informs how many electric shells are consisted in the atom of the element and the group numbers 1, 2 and 13–18 help to determine the number of valence electrons.
Parameter to be determined | Rule |
Number of valence electrons | group 1 and 2: number of valence electrons = number of group groups 13–18: number of valence electrons = number of group – 10 groups 3–12: no rule |
Number of electron shells | number of electron shells = period number |
The change of elements’ properties inthe group and period
Before you watch the teacher conducting the experiment „Lithium reactions with water” formulate a research question and hypothesis. Pay attention to how the elements behave in contact with water and which element causes the strongest reaction. After the experiment, note the observations and conclusions.
Do elements belonging to the same group of the periodic table show similar properties? Do Lithium react with water?
The metals from the first group of the periodic table react with water.
lithium,
sodium,
potassium,
metal pliers,
filter paper,
knife,
three high beakers (500 cmIndeks górny 33).
Pour 150 to 250 cmIndeks górny 33 of distilled water into three beakers.
Place the beakers in a row next to each other.
Take out a piece of metal from the container and put it on dry tissue paper. Cut a piece of metal the size of two grains of rice with a dry knife. Do the same with lithium, sodium and potassium.
Drop each piece of metal into another beaker of water. If possible, do it at the same time.
Observe how the reactions of individual metals with water take place.
Despite the fact that elements belonging to one group have similar properties, elements belonging to one period do not show such similarities. In periods there is a change in the character of elements – from active metals (1st and 2nd group) through active non‑metals to chemically passive noble gases.
Based on the description of the element, determine which element these attributes relate to: it is the most active element among non-metals, has 9 electrons, including 7 valence electrons, has 2 electron shells:
- oxygen
- chlorine
- fluorine
- bromine
Atom of this element has the following electron configuration [2,8,18,7]. This element is:
- chlorine
- bromine
- krypton
- iodine
The ability of the atom to gain electrons ... as the number of the period in the group increases.
- decreases
- increases
- does not change
The ability of atoms to lose electrons... as the number of the period in the group increases.
- increases
- decreases
- does not change
Summary
Period number to which given element belongs is equal to the number of electron shells in its atoms.
Number of valence electrons in atoms of elements belonging to group 1 and 2 is equal to the group number.
Number of valence electrons in atoms of elements belonging to groups 13–18 are obtained by subtracting 10 from the group number.
Elements belonging to the same group show similar properties.
Elements in the same period do not have similar properties.
Explain how following parameters change in particular groups of elements in the periodic table:
density,
size of atomic radius.
Are these values constant or variable? Can you see regularity in the changes of these parameters within the group?
Key words
Structure of the atom, periodic table, valence electrons, location of the element
Glossary
układ okresowy pierwiastków – zestawienie wszystkich pierwiastków chemicznych w postaci rozbudowanej tabeli, uporządkowanych według ich rosnącej liczby atomowej, grupujące pierwiastki według ich cyklicznie powtarzających się podobieństw właściwości, zgodnie z prawem okresowości Dmitrija Mendelejewa.