Exchange reactions
that synthesis and analysis are examples of chemical reactions;
synthesis (coupling) is a reaction that results in only one substance from two or more substances – more complex;
analysis (decomposition) is a reaction that results in at least two or more substances from one compound substance;
digits written before symbols and substance formulas in the chemical reaction equations are stoichiometric coefficients.
to explain what the exchange reaction is;
to save the equations of the exchange reaction along with the selection of coefficients, indication of substances and products;
to present on the models the reaction of the analysis;
to recognize the exchange reactions based on their equations;
to read and write the course of the chemical reaction in words.
What are exchange reactions?
Look at the following reaction equations.
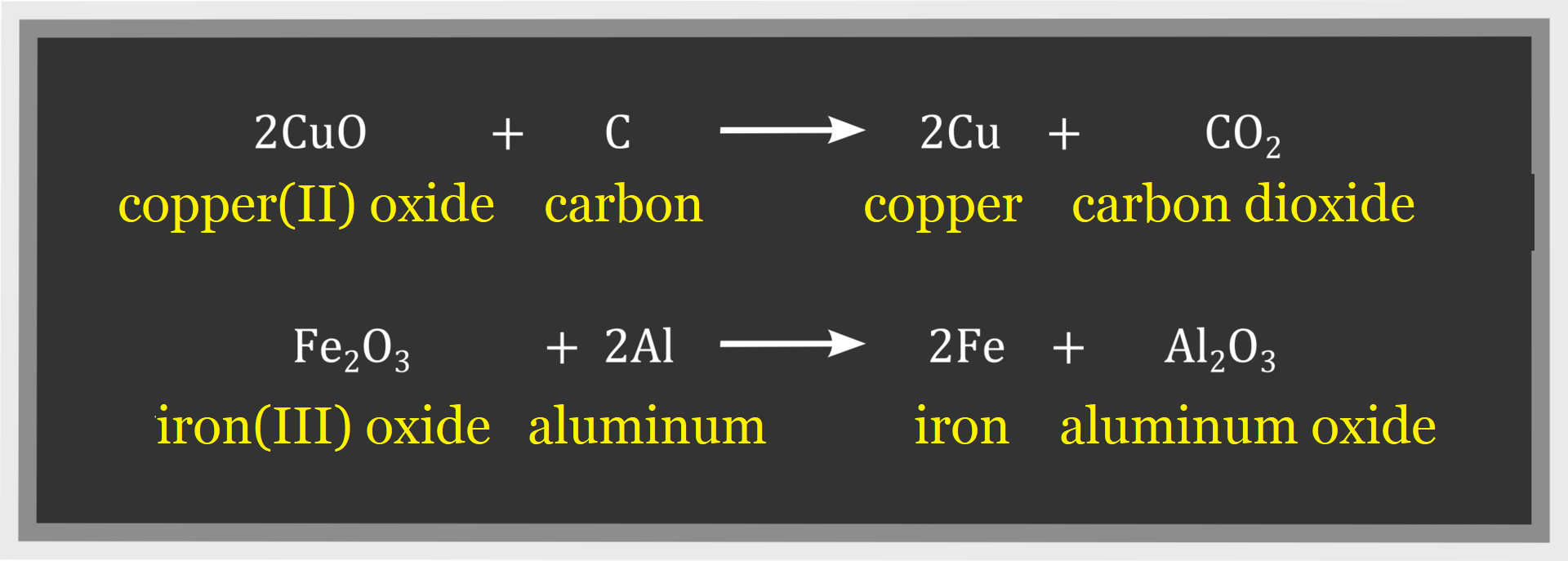
As a result of each of these transformations two other products are created from two different substances.
Equation of reaction | Substances | products |
, | , | |
, | , |
The reactions presented follow the scheme:
These reactions are said to be exchange reactionsexchange reactions.
Before looking at the film „Reaction of lead(II) oxide with carbon”, formulate a research question and hypothesis.

Film dostępny na portalu epodreczniki.pl
Film z przeprowadzenia eksperymentu. Reakcja tlenku ołowiu dwa z węglem. Na porcelanowej płytce umieszczono kawałek węgla drzewnego w którym wydrążono otwór. Następnie przygotowano dokładnie wymieszaną mieszaninę utartego na pył węgla oraz tlenku ołowiu dwa. Mieszaninę umieszczono we wcześniej wydrążonym otworze w węglu drzewnym. Mieszaninę intensywnie ogrzewano bezpośrednio płomieniem palnika. węgiel rozgrzewa się do czerwoności. Po pewnym czasie zaprzestano ogrzewania. Po ostygnięciu na można zauważyć jasny nalot na węglu, a w otworze srebrzyste kuleczki metalu.
What happens during the reaction of lead(II) oxide with carbon? What type of reaction?
Carbon receives oxygen from lead(II) oxide. There is an exchange reaction.
charcoal,
lead(II) oxide,
blow pipe,
spatula,
evaporator,
stand and clamp (circle) for receiving dish,
gas burner.
In a piece of charcoal, make a recess (leave the crumbs selected from the depths - they will be needed later in the experiment).
Put a small amount of lead(II) oxide in the cavity so that it only partially fills them.
Mix the lead(II) oxide with the charcoal crumbs you received while making the cavity.
Ignite the gas burner and use a blower to direct the hot flame to the mixture of lead(II) oxide and carbon.
Watch the changes taking place.
The course of this reaction describes the equation:
Before you see the video „Reaction of iron with copper(II) chloride”, formulate a research question and hypothesis.

Film dostępny na portalu epodreczniki.pl
Nagranie filmowe eksperymentu. Reakcja żelaza z chlorkiem miedzi dwa. Do zlewki z wodą zostaje wsypany zielony proszek, jest to chlorek miedzi dwa. Po wsypaniu zostaje wymieszany łyżeczką. Powstaje niebieski roztwór. Następnie zostaje zanurzony w roztworze żelazny gwóźdź. Po upływie czasu gwóźdź zostaje wyciągnięty. Część gwoździa, która była zanurzona w roztworze ma ceglasto czerwony (miedziany) kolor.
How does iron react with copper(II) chloride? What type of reaction?
The iron will take the place of copper in copper(II) chloride. An exchange reaction will take place.
beaker,
pliers,
measuring cylinder,
a nail (plaque or other object) made of iron,
copper(II) chloride,
water.
Pour a few teaspoons of copper(II) chloride into a beaker.
In this substance, place a nail or other object made of iron.
Observe whether mixed substances react with each other.
Remove the iron object and pour a few dozen cmIndeks górny 33 into the copper(II) chloride water (the resulting solution should be blue).
In the solution obtained, immerse a nail made of iron halfway up its height.
Watch the changes taking place.
The iron displaced copper from an aqueous solution of copper(II) chloride. The copper separated from the solution in the free state. Replacement reaction has taken place:
Which chemical changes can be called exchange reactions?
In this issue, examples of exchange reactions will be presented, as a result of which two other products are formed from two different substrates.
Equation of reaction | ||||
This reaction proceeds according to the scheme:
This reaction can be represented in the form of a schematic:
Complete the chemical equation below:
H2, H3, H2O, Cu
CuO + → + H2O
Chemical transformation, during which at least two products are formed from at least two substrates is called:
- exchange reaction
- combustion
- evaporation
Summary
In addition to the analysis and synthesis reaction, there is a third type of reaction – exchange reaction.
The exchange reaction is a transformation during which the components exchange between the reacting substances takes place. It runs according to the scheme:
.
Keywords
Reduction reaction, exchange reaction
Glossary
reakcja wymiany – przemiana chemiczna, podczas której z co najmniej dwóch substratów powstają co najmniej dwa produkty