Eten and alkenes - hydrocarbons with multiple bonds between carbon atoms
how the general formula of the homologous series of alkanes is created;
how the molecular formula of any alkane with the specified number of carbon atoms is recorded;
which compounds are called saturated hydrocarbons.
what the terms: ***alkenesalkenes***, unsaturated hydrocarbons mean;
to draw structural and semi‑structural formulas of alkenes and alkynes;
to record general formulas of homologous alkenes and alkynes.
Unsaturated hydrocarbons – alkenes
While working on the oil composition, chemists – apart from saturated hydrocarbons – separated a different group of organic compounds, alkenes. AlkenesAlkenes are hydrocarbons in which molecules between carbon atoms have one double bond (multiple). The remaining bonds are single.
Hydrocarbons with at least one multiple bond (double or triple), are called unsaturated.
Unsaturated hydrocarbons, like saturated ones (alkanes), form a homologous series. The name of alkene is formed from the name of an alkane with the same number of carbon atoms in the molecule, adding the ending - ene instead of - ane. The table contains alkenes arranged according to the increasing number of carbon atoms – a homologous series of alkenes.
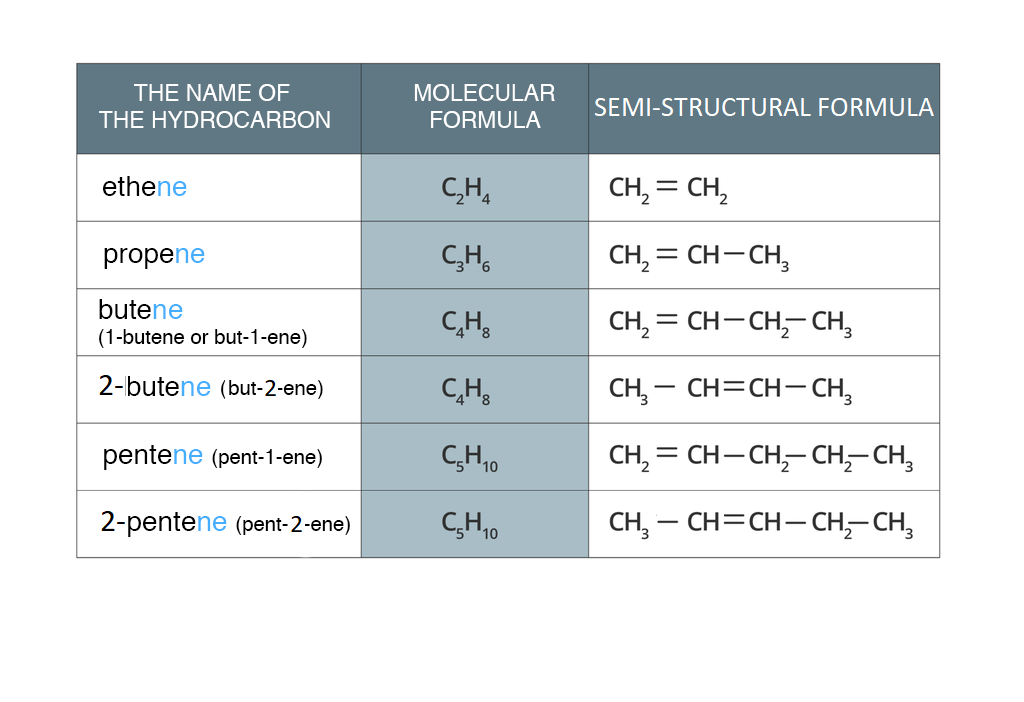
All alkene molecules in the homologous series have twice more hydrogen atoms than carbon atoms. Thus, the general formula of alkenes is:
≥ 2 number of carbon atoms in the molecule.
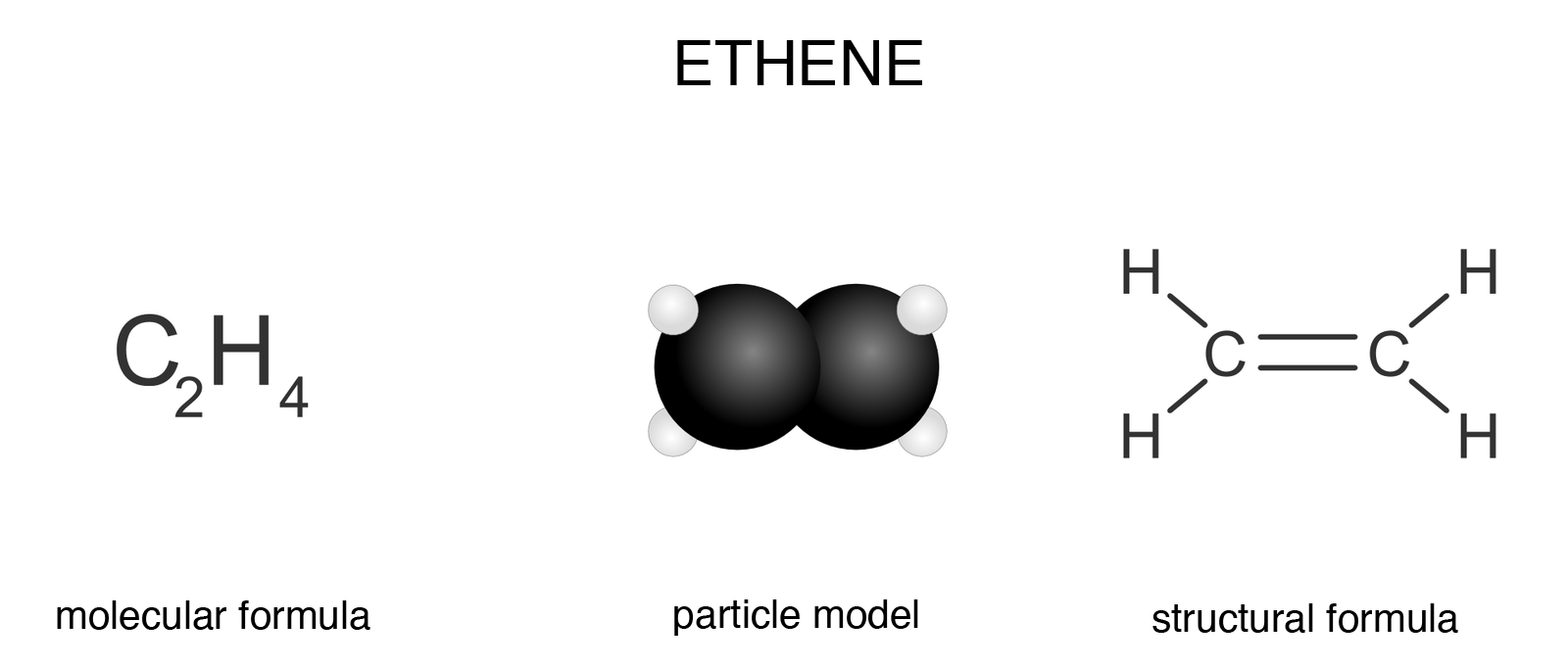
Ethylene
The simplest representative of alkenes is ethene, a compound commonly known as ethylene. Its molecule is composed of two carbon atoms and four hydrogen atoms.
Based on the location of the carbon in the periodic table, we predict that its atom has four valence electrons. Therefore, it is always tetravalent in organic compounds – it can create up to four bonds. In the ethene molecule, the carbon atoms are connected to each other by a double bond (multiple bond). Each of the carbon atoms is connected to two hydrogen atoms.
Design a model of the propene molecule composed of three carbon atoms and six hydrogen atoms.
Before you watch the movie and conduct the experiment „Testing the unsaturated nature of ethene”, formulate a research question and a hypothesis. Write down the observations and conclusions.

Film dostępny na portalu epodreczniki.pl
Film przedstawia badanie nienasyconego charakteru etenu, reaction of the ethene with potassium permanganate. Do przeprowadzenia eksperymentu potrzebne są manganian siedem potasu potassium permanganate, probówka test tube, probówka wypełniona etenem test tube with ethene (gas). Do probówki z etenem wlewamy niewielką ilość roztworu manganianu siedem potasu i mieszamy. Zachodzi reakcja. Roztwór manganianu siedem potasu ulega odbarwieniu. Świadczy to o obecności wiązania wielokrotnego w cząsteczce etenu.
Complete the definition.
Hydrocarbons with at least one ................, double or triple bond are called .......................
Beside the formulas of alkenes, write their names. Use only lowercase letters.
C2H4 – ............
C3H6 – ..............
C4H8 – ............
C5H10 – ..............
Select the butene formula.
- penten
- propen
- buten
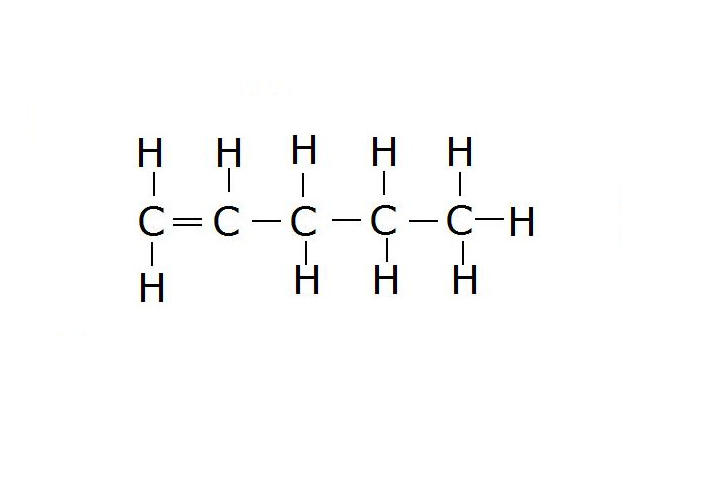
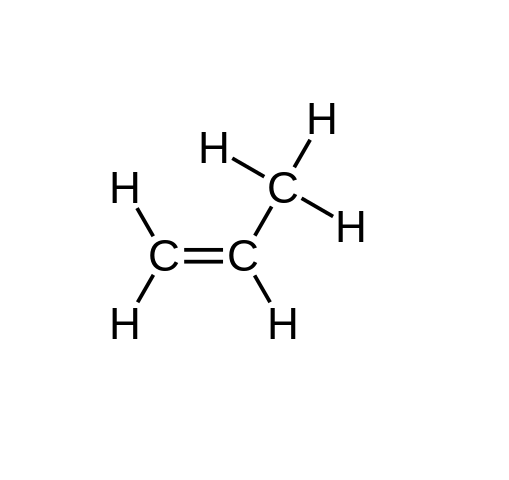
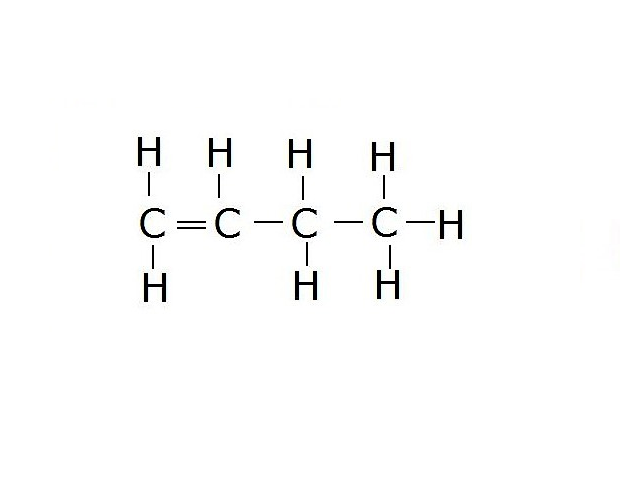
Select true sentences from following.
- Alkene has more than one double bond.
- Etene discolour the aqueous solution of potassium permanganate.
- Alkene with the simplest structure is methene.
- The general formula CnH2n is a formula of the homologous series of alkanes.
- C4H8 is the molecular formula of butene.
Summary
Unsaturated hydrocarbons are hydrocarbons in which molecules between carbon atoms in addition to single bonds there is one double bond (alkenes), and hydrocarbons in which molecules between carbon atoms have one triple bond (alkynes).
Alkenes form a homologous series with the general formula of .
In the ethene molecule, the carbon atoms are connected to each other by a double bond (multiple). Each of the carbon atoms is linked to two hydrogen atoms.
In unsaturated hydrocarbon molecules, multiple bonds can be located between different carbon atoms. This phenomenon is called position isomerism.
Keywords
ethene, ethylene, unsaturated hydrocarbons, alkenes, multiple bonds
Glossary
alkeny – węglowodory nienasycone, związki organiczne zbudowane z atomów węgla i wodoru, zawierające między atomami węgla poza wiązaniami pojedynczymi jedno wiązanie podwójne
izomeria położeniowa – związki o takim samym wzorze sumarycznym, które mogą się różnić położeniem wiązania wielokrotnego oraz położeniem podstawników