The effects of air pollution
what the percentage composition of air is;
how to obtain oxides and write the equations of reactions in which they are obtained;
what properties and applications of gases being air components are;
how the oxygen cycle in nature may be explained.
to list civilization threats;
to discuss what the greenhouse effect is and how smog is formed;
to describe how ozone hole is formed;
to indicate the effects of the decrease in ozone concentration in the stratosphere, the effects of smog and of the greenhouse effect;
to indicate the ways to prevent the increase of ozone hole, smog and the greenhouse effect.
Consequences of air pollution
The most dangerous effects of air pollution are associated with their negative impact on the natural environment. They include:
acid precipitation;
enhanced greenhouse effect;
ozone hole;
smog;
dust;
soil and water pollution.
Acid precipitation
Acid precipitation is atmospheric precipitation (mainly in the form of rain) with an acidic pH – below 5.1. They occur as a result of water droplets absorbing gaseous air pollutants that form acids with tis water, mainly:
sulfur dioxide;
nitrogen oxides, NOIndeks dolny xx;
carbon dioxide;
hydrogen chloride HCl;
hydrogen sulfide HIndeks dolny 22S.
Acid precipitation causes forest dieback. It also affects the acidification of surface waters, which in turn is associated with the extinction of many species of fish. Soil acidification causes the release of toxic aluminum and elution of nutrients and heavy metals. Such precipitation also promotes the destruction of construction materials (stone, concrete, reinforced concrete), and thus the destruction of historic buildings and monuments.

Greenhouse effect
Formulate a research question and a hypothesis before performing the experiment “Greenhouse effect”. Write your observations and conclusions. Pay attention to what is happening with thermometers during the experiment.
What are the consequences of the greenhouse effect?
The greenhouse effect causes temperature increases.
two identical flasks,
two rubber stoppers with embedded thermometers,
lamp,
previously collected carbon dioxide.
Leave the first flask (with air) as a control.
Fill the second flask with carbon dioxide.
Close both flasks with rubber stoppers with embedded thermometers.
Place each of the flasks under a lamp or other source of heat.
Observe the occurring changes, record the indications of the thermometer and make a graph of the temperature change in the flasks depending on the time of exposure.
Ozone hole
In the upper layers of atmosphere, at an altitude between 15 and 50 km, our planet is surrounded by the ozone layer (). It is a natural sunscreen that protects the Earth against excessive ultraviolet (UV) radiation. This radiation is necessary for the production of vitamin D in our body, but its excess may contribute to the reduction of the body's resistance and cause skin diseases and increased cancer morbidity, increase in air temperature and climate changes.
As a result of environmental pollution, especially in the spring over Antarctica and to a lesser extent over the Arctic, the ozone layer becomes thinner and so‑called ozone holeozone hole occurs, through which a significant part of the harmful radiation reaches our planet.
Ultraviolet radiation is invisible and imperceptible to us. Burning and pain caused by its excess is a reaction to skin damage. That's why one should:
limit the time spent in the sun in the summer months at midday;
wear clothing made of thicker, dense fabrics, with long sleeves and legs (the average cotton fabric retains only 20 - 30% of UV radiation);
wear a cap with a visor or a wide‑brimmed hat;
wear sunglasses with UV filters;
use creams with UV filters.
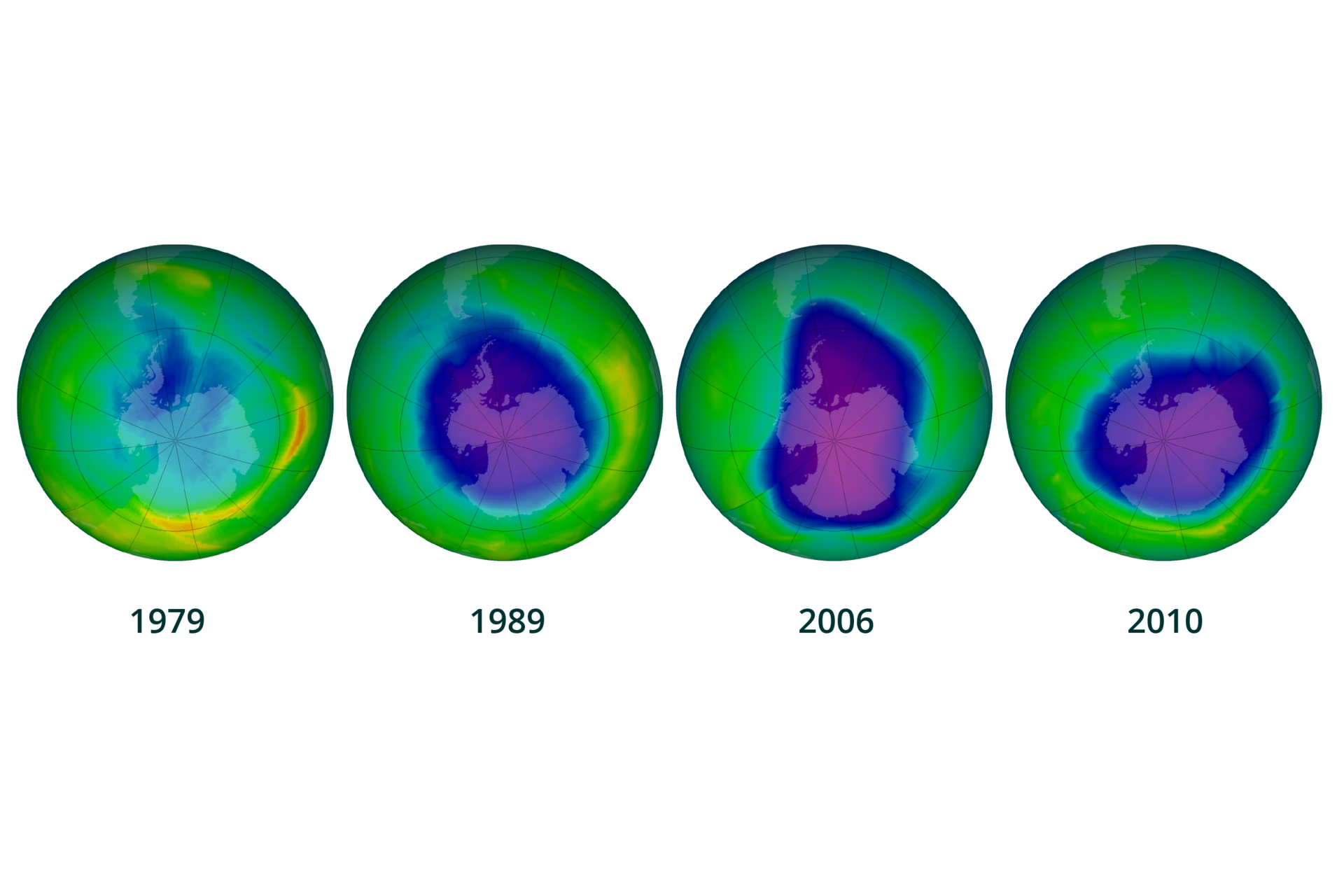
Look for news about ozone hole formation on the Internet, in the e‑textbook and other sources of information and answer the question: What destroys the shield made from stratospheric ozone?
Smog
Pollutants, whose main sources are car fumes, heavy industry and households (heating systems), in combination with windless weather and high humidity – fog, create smogsmog. Smog above the city is particularly dangerous for infants, elderly people, asthmatics, people with respiratory and cardiovascular diseases.
According to the way of formation, place of origin and chemical composition we distinguish the London smog (occurring mainly in the winter months) and the Los Angeles smog (encountered mainly in the summer months).
Expand the subsequent tabs and read the information about smog.
Smog in the past and today in press reports
The word “smog” was created in the English language as a combination of two words: smoke and fog. It surrounds the city like fog, but it is as dangerous as smoke.
1948 Los Angeles Smog
Photochemical smog (also called Los Angeles Smog) is the chemical reaction of sunlight, nitrogen oxides and volatile organic compounds in the atmosphere, which leaves airborne particles and ground-level ozone.
The composition and chemical reactions involved in photochemical smog were not understood until the 1950s.
In 1948, flavor chemist Arie Haagen-Smit adapted some of his equipment to collect chemicals from polluted air, and identified ozone as a component of Los Angeles smog. Haagen-Smit went on to discover that nitrous oxides from automotive exhausts and gaseous hydrocarbons from cars and oil refineries, exposed to sunlight, were key ingredients in the formation of ozone and photochemical smog.
1952 The London Smog
London, 4 December 1952: thick fog began to settle over the city, the breeze stopped, and the air was still. In the result of coal combustion, large amounts of sulfur oxides were released, causing the fog to take on a yellow hue. Soon, hospitals were filled with people suffering from respiratory diseases. In the worst moment visibility in many places was so limited that people did not see their own feet. It is estimated that during the Great Smog of London, the average mortality increased by an additional 4,000-8,000 people - these were mainly infants and the elderly. Air pollution in large industrial cities in Europe was common in the 20th century.
Solid fuels, in particular coal, were often used as fuel in factories and for household heating. In combination with winter conditions and meteorological factors, it happened that very high levels of air pollution persisted over many urban areas for many days, weeks, and even months. Since the 18th century London was notorious for repeated air pollution episodes. Before the 20th century the London Smog became one of the hallmarks of the city, and even gained its place in literature.
2013
In the city of Harbin with the population of 11 million in Northeast China visibility dropped to 10 meters, schools were closed, hundreds of flights and bus lines were canceled, several sections of the highway were closed - all because of the highest smog levels in history.
2014
In the region of Paris and in the north and east of France, a maximum level of air pollution was noted, comparable to that in Beijing and 80% higher than in London and Berlin. Smog threatened the health of people living in Paris. It was caused by the weather - windless, with cold nights and unusually hot daytime for this time of year. The Paris authorities not only called for leaving cars in garages, but also recommended that seniors, children and people with asthma stay home.
Choose the correct answer. Freons contribute to the formation of:
- ozone hole
- smog
- greenhouse effect
- acid precipitation
Choose the correct answer. Freons are the compounds of:
- carbon, fluorine and chlorine
- carbon, fluorine and hydrogen
- fluorine, chlorine and hydrogen
- carbon, chlorine and hydrogen
Choose the correct answer. Ozone can be found in the layer above the Earth’s surface at the height of:
- 10–50 km
- 1–5 km
- 100–500 km
- 50–100 km
Choose the correct answer. The greenhouse effect is caused primarily by:
- carbon monoxide, nitrogen oxides, nitrogen, oxygen
- methane, nitrogen oxides, oxygen, hydrogen
- carbon dioxide, methane, ozone, nitrogen oxides
- carbon monoxide, methane, noble gases, oxygen
Summary
Air pollution has global character. It may originate from natural sources or be the result of human activity.
Air pollutants include, among others, oxides of carbon, oxides of sulfur, oxides of nitrogen, dust and freons.
Unfavorable phenomena caused by atmospheric pollution include: enhanced greenhouse effect, ozone hole, acid precipitation and smog.
Keywords
air pollution, acid precipitation, ozone hole, greenhouse effect, smog
Glossary
dziura ozonowa – potoczne określenie zmniejszenia się ilości ozonu w atmosferze ziemskiej (na wysokości 10–50 km), głównie wokół biegunów
freony – związki węgla z fluorem i chlorem; lotne ciecze lub gazy, bierne chemicznie, niepalne, niezwykle trwałe; znalazły zastosowanie w chłodnictwie, jako środki spieniające używane do produkcji tworzyw sztucznych oraz gaz nośny w aerozolach; są uważane za główną przyczynę powstawania dziury ozonowej
smog – groźna mieszanina zanieczyszczeń powietrza, powstająca głównie w dużych miastach przez osadzenie się tlenku węgla(IV), tlenku siarki(IV) i pary wodnej na cząstkach pyłów i sadzy