The use of sulphates, phosphates and chlorides
that salts are ionic compounds composed of metal cations (or an ammonium cation of the formula ) and anions of the acid residue;
that the salts in the water undergo an electrolytic dissociation.
to recognize salts found in common use and evaluate their impact on the natural environment.
Application of sulphates
Look at the infographics to learn what are the applications of sulphates.
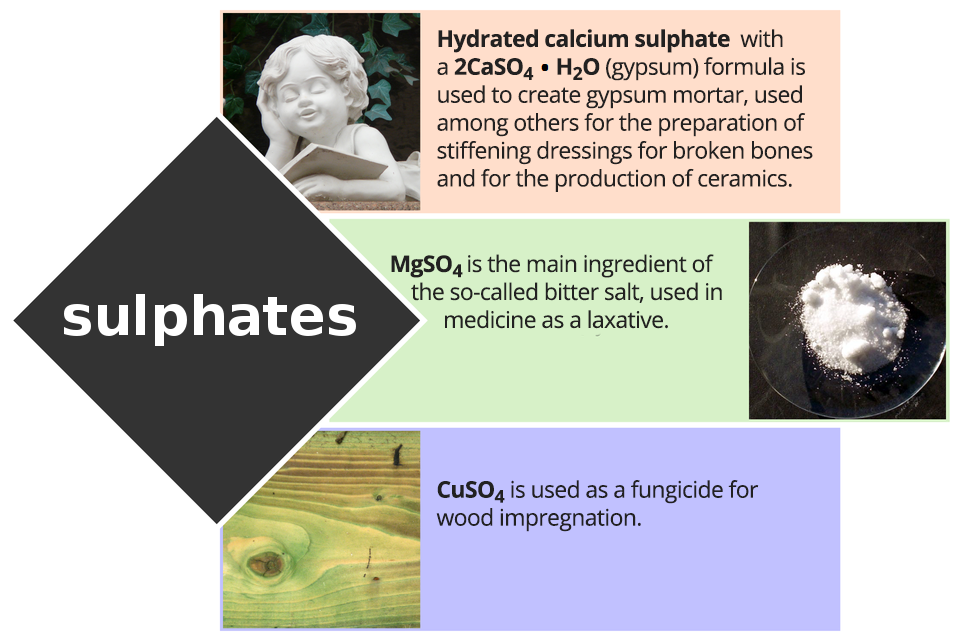
Application of phosphates
Look at the infographics to learn what are the applications of phosphates.

Application of chlorides
Look at the infographics to learn what are the applications of chlorides.

Natural waters contain in their composition dissolved salts derived from the rocks placed on their way. Some of these waters contain a lot of dissolved salts (in an amount of at least 1 g in 1 dmIndeks górny 33), which in appropriate proportions have a beneficial effect on the human body. These waters are called mineral. The most common mineral waters are: .

Solve the crossword.
- Copper(II) sulfate is used for wood impregnation as a…
- A mineral which main ingredient is sodium chloride.
- Chlorides: magnesium, sodium, potassium are ingredients for bathing…
- Sodium phosphate is added to water…
- Salts are components of mineral … used in agriculture
- Natural waters containing salts dissolved in them that come from rocks that meet along the way are water
- Saline is an aqueous solution used in …
- Gypsum is a mineral that consists of calcium …
- NaCl and KNO3 are used for food …
| 1 | ||||||||||||||||
| 2 | ||||||||||||||||
| 3 | ||||||||||||||||
| 4 | ||||||||||||||||
| 5 | ||||||||||||||||
| 6 | ||||||||||||||||
| 7 | ||||||||||||||||
| 8 | ||||||||||||||||
| 9 |
Check the illustrations on the use of chlorides.
- Production of mirrors
- Bath and cosmetic salts
- Food salt
- Production of fertilizers




Adjust the salt application to the appropriate groups.
production of cosmetic salts, e.g. bath salts, production of food salt, production of drugs based on physiological saline, production of ceramics, production of laxatives, production of bone stiffening dressings, production of protective preparations for wood, production of artificial fertilizers with phosphorus, production of water softeners
| sulfates | |
|---|---|
| chlorides | |
| phosphates |
Answer the questions, then reverse the tab by clicking on it and check the correctness of the answer.
| Potassium chloride has the formula... | KCl |
| Salt used for the production of potassium ions containing fertilizers | Potassium chloride |
| It is used to create plaster mortar | Plaster gypsum |
| The main ingredient of bitter salt | Magnesium sulfate |
| Copper(II) sulfate has the formula… | CuSO4 |
| This salt is used for the production of wood preservatives | Copper sulfate |
| You can find this salt in water softeners | Sodium phosphate |
| Common components of bath salts are… | KCl, NaCl, MgCl2 |
| The main ingredient of rock salt is ... | Sodium chloride |
| Lime has the formula… | 2CaSO4•H2O |
Summary
Salts occur in nature in a dissolved form in natural waters or form minerals
Nitrates and phosphates are used, among others for the production of synthetic fertilizers.
Calcium carbonate is, for example, a raw material for the production of building materials.
Sodium chloride is used, among others for food, in the pharmaceutical industry and for snow‑covered streets in winter.
On the internet you can find websites of stores selling so‑called agents for deacidification of the body. These are dietary supplements containing vitamins or plant extracts, but these are also agents with very poor composition – they contain one of the salts you know. Find out what salt is it. What types of known food contains it? Write the answer in a notebook.
Key words
sulphates, chlorides, phosphates, application of salt
Glossary
eutrofizacja – zjawisko polegające na zwiększeniu żyzności wód w zbiorniku wodnym