Water - an amazing liquid
what distinguishes the states of substance matter and how the particles are arranged in each of them;
how a change in temperature affects the state of the substance, including water.
describe the properties of different states of matter of water;
discuss the importance of water for living organisms;
recognize the states of matter of water in nature.
Nagranie dostępne na portalu epodreczniki.pl
Wysłuchaj nagrania abstraktu i zastanów się, czego jeszcze chciałbyś się dowiedzieć w związku z tematem lekcji.
What are the properties of water?
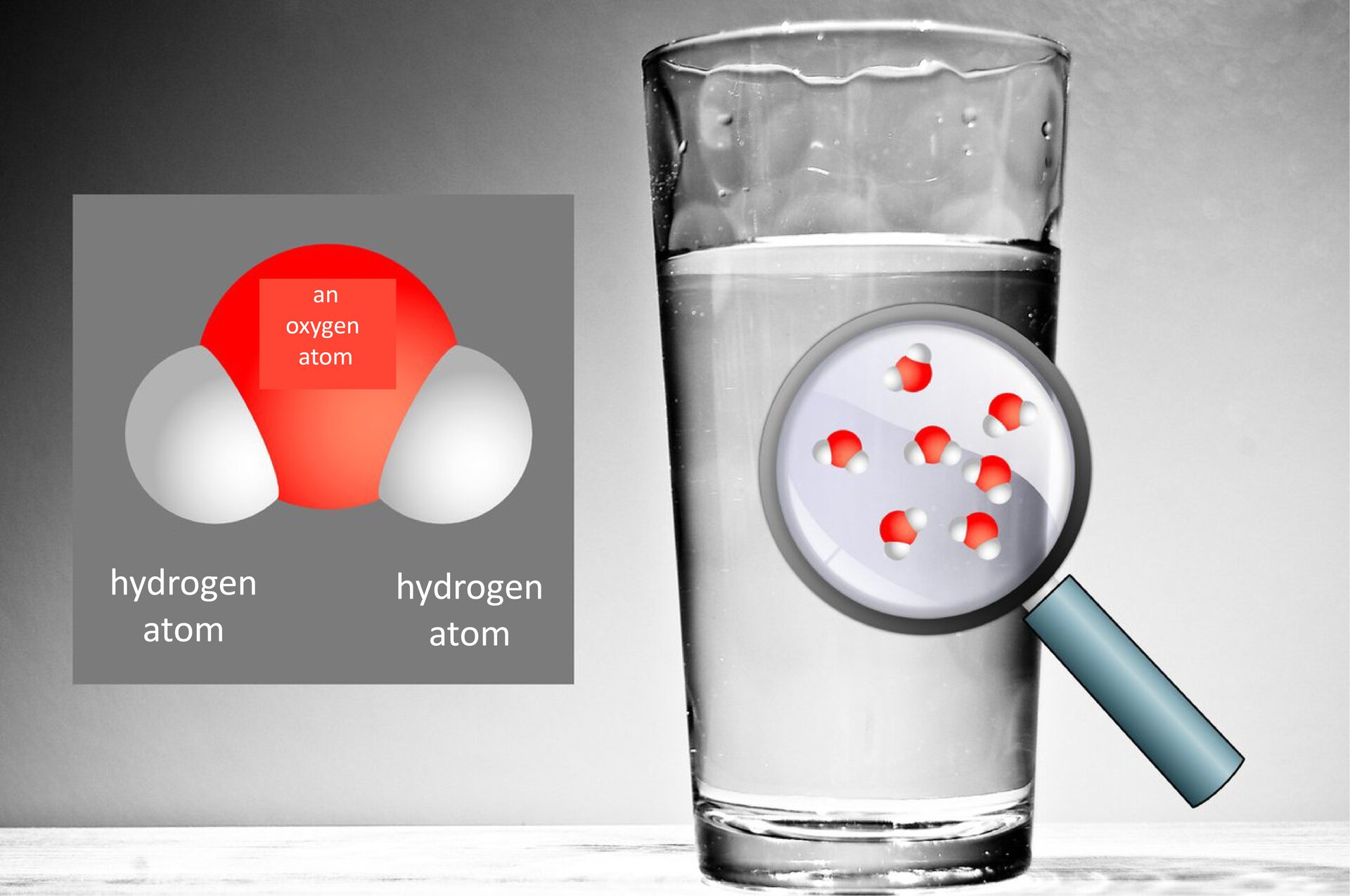
Clean waterwater has no taste, fragrance or color – only in large water reservoirs looks light blue. It is a liquid at room temperature. It freezes at 0°C and boils at 100°C. Many substances dissolve in it. In each state of matter it has its own separate name: in the solid state we call it ice, in the liquid state – simply water, and in the gas state – water vaporwater vapor.
Conducting processes of changing the water's state.
container for ice cubes,
glass,
a small pot (or frying pan),
freezer,
stove,
water
SolidificationSolidification of water. Fill the ice container with water. Put the container in the freezer (there is a negative temperature). The next day, take out the container and see what happened.
MeltingMelting ice. Take out some ice cubes from the container and put them in a glass. Put it at room temperature, e.g. on the table. Wait a few minutes. Check what happened.
Boiling water. Pour water into a small pot or pan. Warm the pot on the stove. The dish will warm up to several hundred degrees Celsius. Be careful not to burn yourself. Watch what is happening with the water.
Temperature changes cause the water to change its state of matter.
Properties of water may vary if it contains additives. We all know the term „salt water” – it is the water in the seas and oceans. Various substances, mainly salt, are dissolved in it. How, apart from the taste does sea water differ from „fresh water”, is it containing smaller amounts of salt? It is thicker, so that small objects can be kept on its surface. Maybe you've seen pictures from the Dead Sea, where you can see people floating on the sea surface effortlessly? This is due to the salinity – in the Dead Sea it is extremely large and amounts to 26% at the surface. For comparison, the salinity of the Baltic Sea is on average 0.7%.

Examine what is the difference between salt water and fresh water.
glass,
water,
salt,
teaspoon,
ink.
Fill the glass half with water.
Put 4 teaspoons of salt into it and mix thoroughly.
Add a few drops of ink so that the salted water differs from the pure color.
Carefully pour water from the tap with a teaspoon so that it flows down the side of the glass. What are you seeing?
If the fresh water is poured carefully, it does not mix with the salt, but settles on its surface. This means that its density is less than the salt water density.
Where is the water?
Water occurs in our environment in all three states of matter. The fact that it exists in the form of liquid and ice, no one needs to convince us. But how to tell if there is water vapor around us? It's enough to cool it to condensecondense.
Checking for water vapour in the air.
glass,
ice cubes.
Fill the glass with ice cubes.
Wait a moment, carefully looking at the glass. What are you seeing?
Touch the outer surface of the glass. What do you feel?
If you feel moisture when touched, it means that water has appeared on the sides of the glass. There is water vapor in the air surrounding us. Touching the cold sides of the glass, it cools and condenses.
Water plays a very important role in the life of organisms, including humans. It is necessary that various processes take place in our body, such as breathing or blood circulation, thanks to which we function properly. In our climate, an adult needs around 2.5 l of water a day. As a good solvent, this substance is also needed in many areas of human activity. During daily activities (eg. Washing, dishwashing), we consume up to 300 liters of water per day. Water is also used in industry (eg for the production of 1 kilo paper, 400 - 800 l of water is used) and agriculture – for watering crops.
Temperature expansion of water
Water, like all substances, reacts to changes in temperature – it therefore undergoes thermal expansion. However, in the case of water this phenomenon is different than in the case of other substances.
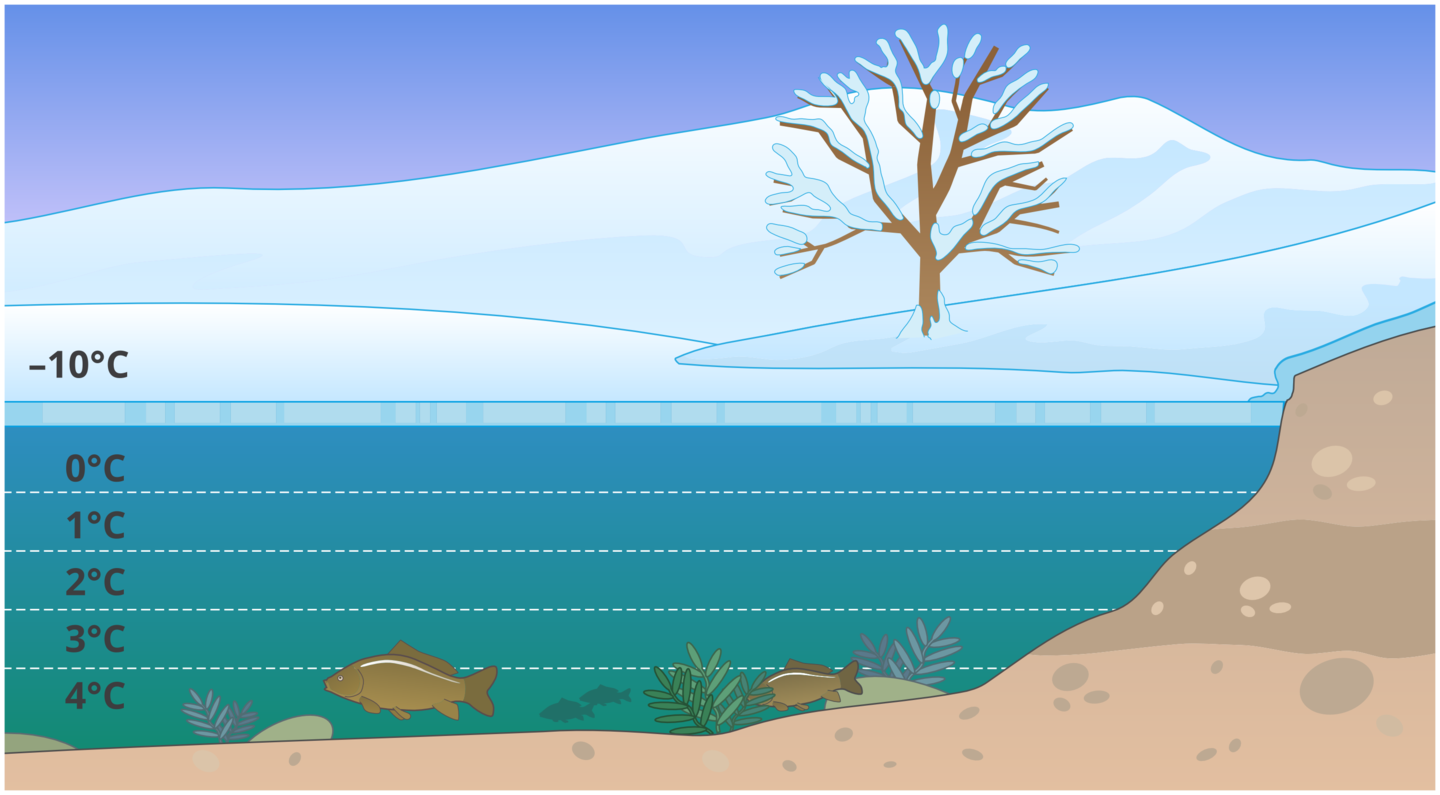
Density of water and ice
Usually densitydensity of the substance increases with the temperature drop. This means that if the temperature drops, the body with a given mass takes up a smaller volume. However, water has it’s highest density at 4°C. By lowering the temperature below this value, the water increases the volume again.
Before you watch the film „Water temperature expansion”, write down the research question and the hypothesis. Make notes while watching the movie, and finally add conclusions.

Film dostępny na portalu epodreczniki.pl
Nagranie eksperymentu. Do plastikowej butelki nalano wody. Zaznaczono markerem jej poziom. Eksperymentator włożył butelkę do zamrażarki. Poziom zamrożonej wody jest wyższy od tej w stanie płynnym. Zaznaczono markerem wyższy poziom.
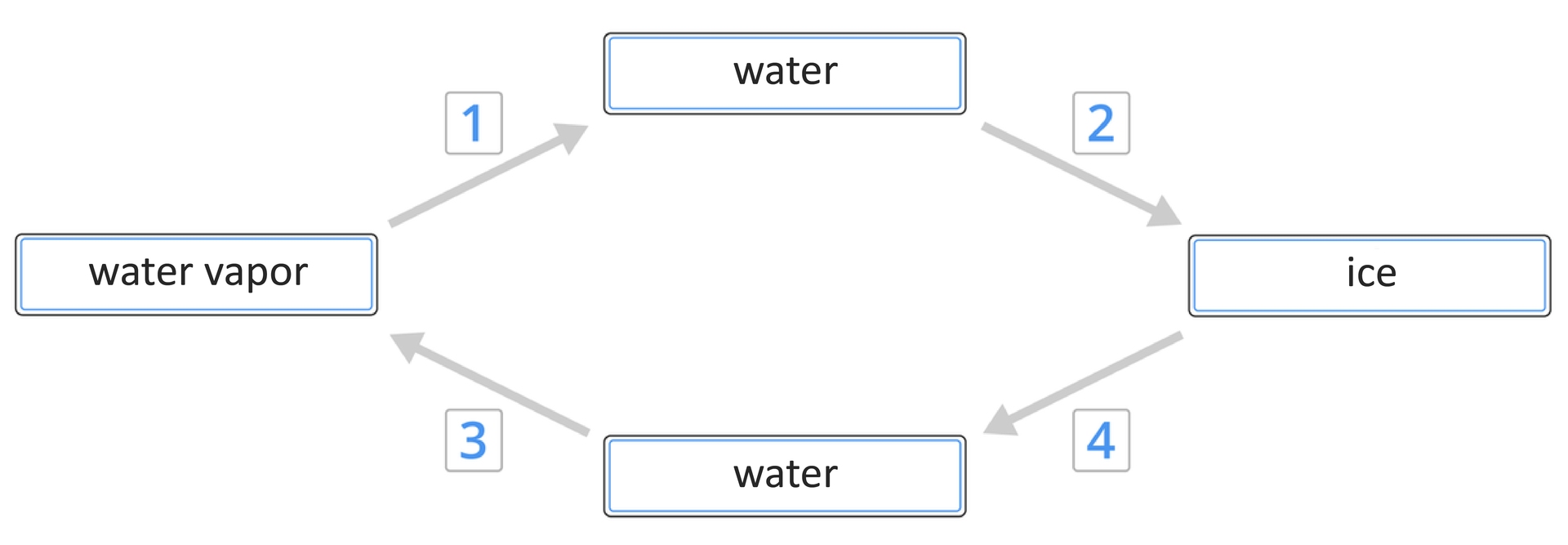
Based on the graphics attached above, combine the numbers denoting the processes with the corresponding names of these processes.
condensation, solidification, evaporation, melting
| 1 | |
| 2 | |
| 3 | |
| 4 |
Observed situations are associated with changes in water states. Assign their descriptions to the names of the relevant processes.
Often in the morning in the meadow there is dew., When you cook soup in a pot, some of it decreases., In winter, ponds and lakes are covered with a layer of ice., With properly prepared ingredients when inserted into the refrigerator arise delicious ice cream., When cooking on the inside of the lid placed on a pot, drops of water are collected., Hanged laundry dries after a while., A delicious ice sorbet left on a warm day in the kitchen on the table becomes after a while a simple drink., On a sunny winter day, drops of water fall from the snow-covered roof.
| melting | |
|---|---|
| solidification | |
| evaporation | |
| condensation |
Summary
In our environment, depending on the temperature, water can occur in three states – solid, liquid and gas.
Water plays an important role in nature and in human life.
Water has the highest density at about 4°C (it is the heaviest). Ice has a lower density (it is lighter) than cold water.
Keywords
water, density, evaporation, melting, solidification, condensation
Glossary
woda – bezbarwna i bezwonna substancja, w temperaturze pokojowej ma postać cieczy; potocznie określenie to stosowane jest wyłącznie do wody w stanie ciekłym
gęstość – właściwość substancji, która określa ilość masy zawartej w jednostce objętości
parowanie – zmiana stanu skupienia z cieczy w gaz; zachodzi jedynie na powierzchni cieczy i w różnych temperaturach
topnienie – zmiana stanu skupienia z ciała stałego w ciecz
krzepnięcie – zmiana stanu skupienia z cieczy na ciało stałe
skraplanie – zmiana stanu skupienia gazu w stan ciekły