Noble gases
what is the relationship between the structure of the element's atom and its position in the periodic table;
how to indicate the location of nitrogen in the periodic table of elements;
what are the examples of nitrogen applications in everyday life;
how to discuss the nitrogen cycle in nature.
to indicate the location of noble gases in the periodic table of elements;
to list elements included in the group of noble gases;
to describe the properties of noble gases;
to give examples of the use of noble gases in the immediate vicinity.
Noble gases
The air components are argon and other elements forming the 18th group in the periodic table. Noble gases include: heliumhelium (He, Latin helios), neonneon (Ne, Latin neon), argonargon (Ar, Latin argon), krypton, xenon, radon. Stable electron configuration of valence shell ensures that these gases are the elements with the lowest chemical activity (noble gases). Therefore, although all of them are gases, their atoms do not bind into particles. They occur in the form of single atoms. Noble gases are used, among others, in medicine and in the lighting industry.
Chemists try to combine noble gases with other substances. In the 1960s compounds containing xenon, krypton and radon were obtained. It has recently been reported that argon - the most common noble gas - can permanently bond with fluorine and hydrogen. To this day, no bonding with helium or neon has been obtained.
In the group of the periodic table of elements there is also a synthetic element oganesson with the atomic number Z = 118. We owe this discovery to the experiments carried out in the Flerova Nuclear Reaction Laboratory (Dubna, Russian Federation) by a team of scientists from the United Nuclear Research Institute in the Russian Federation and the U.S. Lawrence Livermore National Laboratory. One ununoctium atom was obtained in the summer of 2002, and two more in 2005. In the past, it was thought that oganesson would be a gas under standard conditions, but current predictions indicate a constant state of aggregation under these conditions.
Look at the interactive illustration and remember the uses of noble gases.
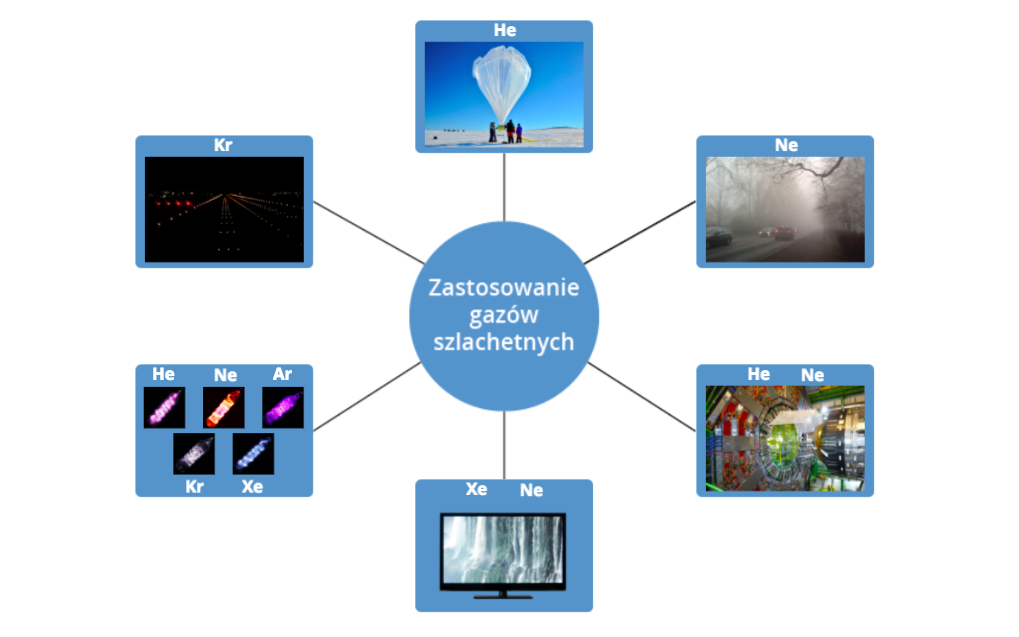
1. Krypton is used for airport runway lights
2. Helium It is used, among others, for filling balloons and meteorological probes.
3. Neon is used in fog lamps
4. Helium and neon (in liquid state) are used to cool devices operating at very low temperatures (close to absolute zero). Great Hadron Collider at CERN's European Center for Nuclear Research near Geneva is cooled in this way.
5. Neon and xenon in plasma TV screens
6. Neon and other noble gases are used to fill lamps
7. Use of noble gases
Create a multiple-choice test based on today's lesson. Then exchange your questions with a friend or classmate.
Question: ...
- ...
- ...
- ...
- ...
Summary
Noble gases show the least chemical activity among all known elements. This property is related to the durability of the electronic helium configuration.
Noble gases are used, among others in lighting technology.
In the bedroom you want to hang a logo with your name. Provide the color of the logo and the type of gas, you will use to get the color.
Keywords
noble gases, helium, argon, neon, krypton, xenon, radon
Glossary
pierwiastek chemiczny z grupy helowców o największym rozpowszechnieniu na Ziemi; jego zawartość w atmosferze wynosi 0,94% (procenty objętościowe); stosuje się go w procesach chemicznych wymagających obojętnego środowiska, np. podczas spawania, do wypełniania przestrzeni zespolonej w oknach oraz w mieszaninie do wypełniania żarówek razem z azotem
pierwiastek chemiczny z grupy helowców; po wodorze drugi najbardziej rozpowszechniony pierwiastek chemiczny we wszechświecie; jest niepalny; stosuje się go do napełniania balonów, jako czynnik chłodzący w reaktorach jądrowych, a także składnik mieszaniny z tlenem w butlach tlenowych dla nurków
pierwiastek chemiczny z grupy helowców; stosuje się go do produkcji lamp jarzeniowych (czerwona barwa), w urządzeniach elektronicznych