Non-metal – properties and application
what are the properties of matter in various states;
what are symbols of chemical elements and how to use them;
how to determine and distinguish between physical and chemical properties;
where non‑metals are located in the periodic table of elements;
what safety rules should be followed in the school chemical laboratory.
what is the application of non‑metals in everyday life;
to plan experiments to examine the properties of non‑metals;
to distinguish metals from non‑metals based on properties;
to plan experiments to compare the properties of metals and non‑metals;
compliance with safety rules when performing chemical experiments.
Non‑metals properties
Before you doing the experiment „Testing the properties of non‑metals”, write down the research question and hypotheses. Write down observations and conclusions.
sulfur,
coal in the form of graphite,
red phosphorus,
chlorine,
white phosphorus,
bromine,
tweezers,
paper,
hammer,
goggles
Determine the state of matter and the color of the substance.
Place non‑metal on the paper, then hit it with a hammer.
Write down observations.
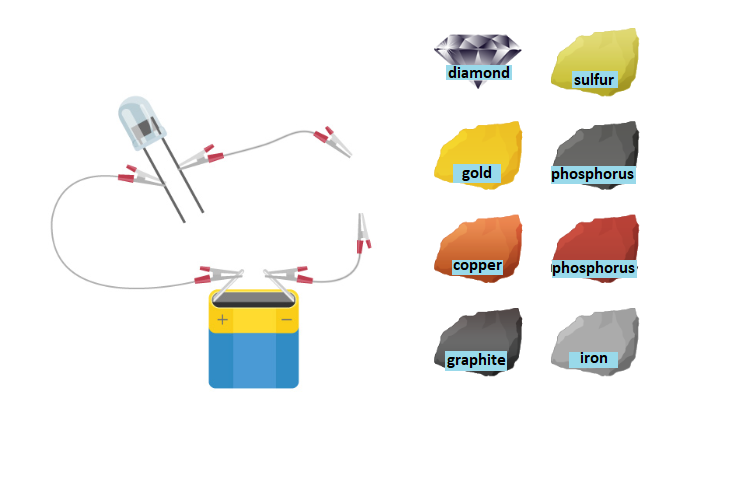
Before you doing the experiment „Testing the electrical conductivity of non‑metals”, write down the research question and hypotheses. Make a note of observations and conclusions.
1. Non-metals are electrically conductive.
2. Non-metals do not conduct electricity.
battery,
light bulb,
electric cables,
metal samples, e.g. iron, copper and gold,
non‑metal samples, e.g. sulfur, carbon in the form of gafit and diamond.
Insert a sample of examined substances under test in a properly assembled circuit.
Observe what is happening with the bulb.
Non‑metal (symbol) | Colour | State of matter | Gloss | Density [kg/m³] | Melting point [°C] | Boiling point [°C] |
carbon () (graphite) | grey‑black | solid | none | 2300 | 4000 (sublimation) | – |
carbon () (diamond) | colourless | solid | none | 3500 | 4800 (sublimation) | – |
sulphur () | yellow | solid | none | 2070 | 119 | 445 |
phosphorus () (white) | white | solid | none | 1820 | 44 | 280 |
silicon () | dark grey | solid | metallic | 2330 | 1417 | 3280 |
chlorine () | green‑yellow | gas | none | 3.2 | –101 | –34 |
bromine () | dark brown | liquid | none | 3119 | –7 | 59.5 |
iodine () | grey‑black | solid | metallic | 4940 | 114 | 185 |
Using the Internet, a book and e‑textbook, search and compile information on the use of non‑metals. You can also use the drop‑down table.
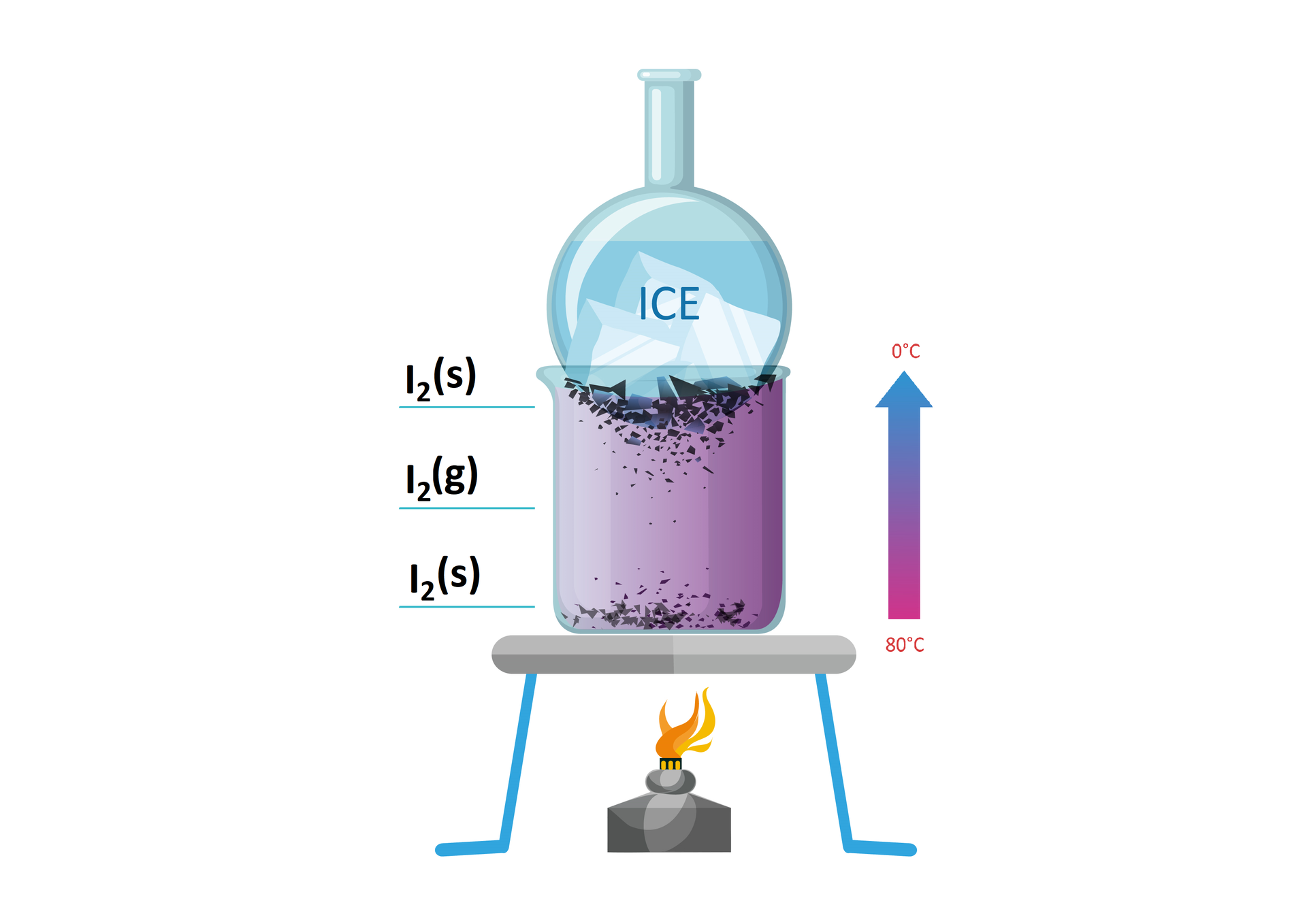
Before you doing the experiment „Sublimation and deposition of iodine”, write down the research question and hypotheses. Make a note of observations and conclusions.
beaker,
tripod,
round bottom flask,
gas burner,
lighter
metal mesh
cold water
iodine crystals
Place a burner under the tripod and a metal mesh on the tripod.
Place a beaker with iodine crystals on the tripod.
Place a round bottom flask with cold water on the beaker with iodine, ignite the burner and heat.
Observe the changes taking place in the beaker and after some time on the surface of the round bottom flask (on the side of the beaker).
Summary
We divide chemical elements into metals and non‑metals.
Non‑metals form the right part of the periodic table, the exception is hydrogen, which is in the first group.
Non‑metals occur in all states of matter, have different colours, can have characteristic odours, have different boiling and melting points, are usually weak electrical and heat conductors. Carbon in the form of graphite, graphene and black phosphorus conduct electricity and heat despite the fact that these are non‑metals, the diamond only conducts heat, these are mostly gases at room temperature.
Non‑metals have different applications: in medicine, pharmacy, in the production of fertilizers, dyes, in the food industry.
Present a comparison of properties of metals and non‑metals in the form of a table.
Keywords
non‑metals, state of matter, allotropes, properties of non‑metals, non‑metals application
Glossary
niemetale – pierwiastki chemiczne, które w odróżnieniu od metali źle przewodzą prąd elektryczny (z wyjątkiem grafitu, grafenu i fosforu czarnego) i ciepło (z wyjątkiem diamentu, grafitu i fosforu czarnego); w stanie stałym są na ogół kruche, bez metalicznego połysku (z wyjątkiem jodu, krzemu, grafitu)