Structure of the atom
how the atom is built and what it is made of,
be able to discuss the properties of the atomic nucleus components in English.
Answer the introductory questions for the lesson.
What is matter consist of?
What is the electric charge? What properties does an electric charge have?
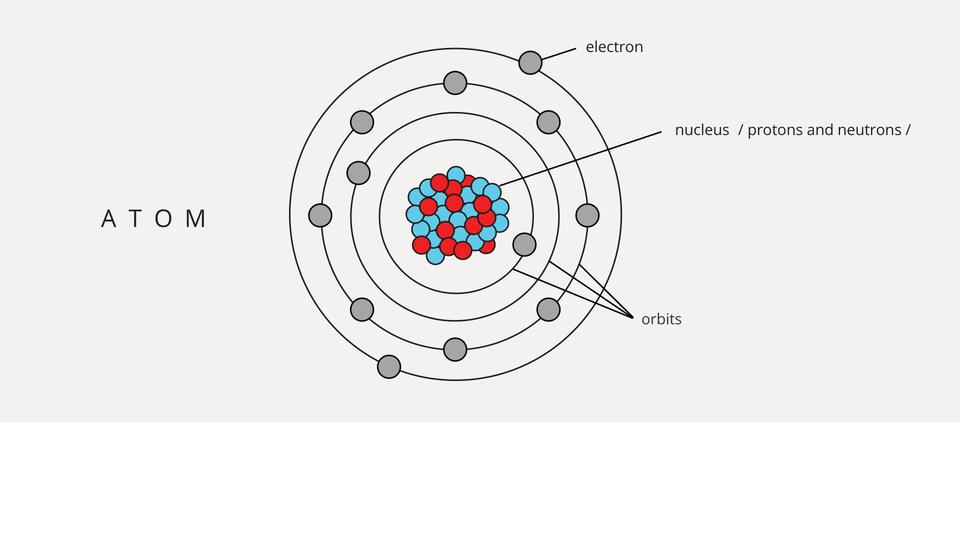
The atomatom is made up of a positively charged nucleus and a negatively charged electronelectron sphere around it.
Electrons circulating at different distances from the nucleus form electron shells. The number of shells is variable. However, we are able to determine how many electrons are on a given shell.
Zasób interaktywny dostępny pod adresem https://zpe.gov.pl/a/DtGtdrI7o
From the available materials, create a model of the oxygen atom.
Use the information that there are 8 neutrons and 8 protons in the nucleus of the atom, and the electrons are circling around the nucleus: 2 in the closer orbit and 6 in the further orbit.
Summary
Atoms consist of the nucleus and the electrons surrounding it. In the nucleus there are nucleons: protons and neutrons.
Neutrons are electrically neutral particles; protons carry a positive electric charge and electrons - negative.
In an electrically neutral atomatom, the number of protons and electrons is the same. In this case, the total charge of protons and electrons is zero.
Atoms with the number of electrons different from the number of protons are called ions that are atoms having an electric charge.
The properties of atoms depend mainly on the number of protons in the atomic nucleus. Groups of atoms with the same number of protons in the nucleus and different number of neutrons are referred to as the isotopes of a given elementelement (determined by the number of protons).
Atoms are the basic elements building matter from the point of view of chemistry and remain the smallest particles distinguished by chemical methods. They do not change in chemical reactions.
Exercises
Determine which sentences are true.
- Atom - is the smallest part of the chemical element that maintains its chemical properties.
- Atoms of the same elements have different properties (size, mass, colour). Atoms of the same elements join together to form molecules.
- Atoms of various elements have the same properties, e.g. size, mass, and can combine with each other to form a chemical compound.
- The structure of an atom - an atom consists of a nucleus in which there is a positively charged proton and a neutron that has no charge. Outside of the nucleus, electrons with a positive charge circulate around closed curves called orbits or shells.
- The electrons on the last orbits are called valence electrons. Atom in the ground state is electrically neutral, i.e. it has as many electrons as protons. The structure of the atom resembles the Solar System, in the middle is the Sun (nucleus), and beyond it the planets (electrons) circulate.
If n is the number of the orbit (counting from centre of the atomatom) then the maximum number of electrons that can fit in such an orbit is expressed by the formula:
where:
n - the number of another orbit,
k - the number of electrons on the n‑orbit.
Orbits are usually marked with capital letters K, L, M, N, O, P, R, ... etc.
Using the periodic table of elements, complete the table below specifying the electronelectron configurations. Give the right number of electrons to the name of each orbit.
Atomic number A | Element | K | L | M | N | O |
24 | chromium | 2 | 8 | 14 | 0 | 0 |
palladium | ||||||
uranium | ||||||
carbon | ||||||
copper |
Nowadays the view about the structure of an atom differs significantly from the first theory of it. Use the knowledge available to you and point out some basic differences. Write a short note in English on this topic.
Indicate which pairs of expressions or words are translated correctly.
- atom - atom
- proton - element
- pierwiastek - proton
- orbita elektronu - electron orbit
- elektron - electron
- orbita elektronu
- atom
- elektron
- atom
- electron orbit
- electron
- proton
- pierwiastek
- element
- proton
Glossary
atom
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: atom
elektron
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: electron
orbita elektronu
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: electron orbit
pierwiastek
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: element
proton
Nagranie dostępne na portalu epodreczniki.pl
wymowa w języku angielskim: proton
Keywords
atomatom
electronelectron
protonproton